XV WIDER CARIBBEAN
XV-49 Caribbean Sea LME
XV-50 Gulf of Mexico LME
XV-51 Southeast U.S. Continental
Shelf
LME
656
XV Wider Caribbean
XV Wider Caribbean
657
XV-49 Caribbean Sea LME
S. Heileman and R. Mahon
The Caribbean Sea LME is a tropical sea bounded by North America (South Florida),
Central and South America and the Antilles chain of islands. The LME has a surface
area of about 3.3 million km2, of which 3.89% is protected, and contains 7.09% and
1.35% of the world's coral reefs and sea mounts, respectively (Sea Around Us 2007).
The average depth is 2,200 m, with the deepest part, the Cayman Trench, at 7,100 m.
Most of the Caribbean islands are influenced by the nutrient-poor North Equatorial
Current that enters the Caribbean Sea through the passages between the Lesser
Antilles. A significant amount of water is transported northwestward by the Caribbean
Current through the Caribbean Sea and into the Gulf of Mexico, via the Yucatan Current.
Run-off from two of the largest river systems in the world, the Amazon and the Orinoco,
as well as numerous other large rivers dominates the north coast of South America
(Müller-Karger 1993). A book chapter and reports pertaining to this LME have been
published by Richards & Bohnsack (1990) and UNEP (2004a, 2004b, 2006).
I. Productivity
The Caribbean Sea LME can be considered a Class II, moderate productivity ecosystem
(150-300 gCm-2yr-1). There is considerable spatial and seasonal heterogeneity in
productivity throughout the region. Areas of high productivity include the plumes of
continental rivers, localised upwelling areas and nearshore habitats such as coral reefs,
mangroves and seagrass beds. Relatively high productivity occurs off the northern coast
of South America where nutrient input from rivers, estuaries and wind-induced upwelling
is greatest (Richards & Bohnsack 1990). The remaining area of the LME is mostly
comprised of clear, nutrient-poor waters.
The Wider Caribbean Region is a biogeographically distinct area of coral reef
development within which the majority of corals and coral reef-associated species are
endemic (Spalding et al. 2001, Wilkinson 2002), making the entire region particularly
important in terms of global biodiversity. Among the LME's coral reefs is the Meso-
American Barrier Reef, the second largest barrier coral reef in the world. There have
been yearly migrations of marine mammals such as the humpback, sperm and killer
whales. Manatees not as common as they once were along many of the river mouths.
Sea turtles, such as hawksbill, green and leatherback nest on beaches within this LME.
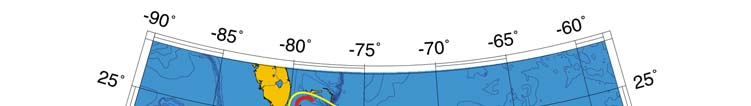
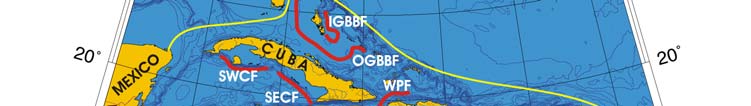
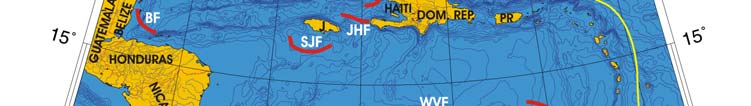
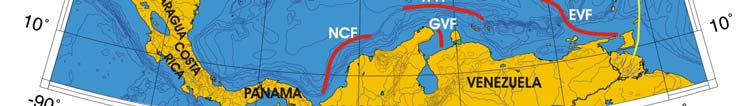
Oceanic Fronts (Belkin et al. 2008)( Figure XV-49.1): In the southern Caribbean Sea,
fronts are generated by coastal wind-induced upwelling off Venezuela and Colombia at
75°-78°W, 70°-75°W, and 62°-66°W. A 100-km-long front dissects the Gulf of Venezuela
along 70°40'W, likely caused by the brackish outflow from Lake Maracaibo combined with
coastal upwelling. Two shelf-break fronts off Cuba encompass two relatively wide shelf
areas off the southern Cuban coast, east of Isla de la Juventad (83°W) and along the
Jardines de la Reina island chain (79°-80°W), both best developed in winter. The
Windward Passage Front between Cuba and Hispaniola (73°W) separates the westward
Atlantic inflow waters moving into the Caribbean in the western part of the passage from
the Caribbean outflow waters heading eastward in the eastern part of the passage. A
200-km-long front in the Gulf of Honduras peaks in winter, likely related to a salinity
differential between the Gulf's apex and offshore waters caused by high precipitation in
southern Belize (Heyman & Kjerfve 1999).
658
49. Caribbean Sea LME
Figure XV-49.1. Fronts of the Caribbean Sea LME. Acronyms: BF, Belize Front; DOM.REP., Dominican
Republic; EVF, East Venezuela Front; GVF, Gulf of Venezuela Front; IGBBF, Inner Great Bahama Bank
Front; JHF, Jamaica-Haiti Front; NCF, North Colombia Front; OGBBF, Outer Great Bahama Bank
Front; PR, Puerto Rico (U.S.); SECF, Southeast Cuba Front; SJF, South Jamaica Front; SWCF,
Southwest Cuba Front; WPF, Windward Passage Front; WVF, West Venezuela Front. Yellow line, LME
boundary. After Belkin et al. (2008).
Caribbean Sea LME SST (Belkin 2008)(Figure XV-49.2):
Linear SST trend since 1957: 0.03°C.
Linear SST trend since 1982: 0.50°C.
The Caribbean Sea went through three phases over the last 50 years: (1) cooling until
1974; (2) cold phase with two cold spells of 1974-1976 and 1984-1986; (3) warming since
1986. Using the year of 1985 as a true breakpoint, the post-1985 warming amounted to
>0.6°C over the last 20 years. Both cold spells were synchronous with cold events
across the Central American Isthmus, in the Central American Pacific LME. The first
cooling period was interrupted by a major warm event (peak) of 1968-1970, when SST
reached its all-time maximum of 28.2°C in 1969. This event was confined to the
Caribbean Sea. None of the adjacent LMEs experienced a pronounced warming in
1968-1970. If the warm event of 1968-1970 cannot be explained by anomalous
atmospheric conditions, the reason should be in the open ocean east of the Caribbean
Sea, in the trade winds zone, where the Canary Current LME experienced a warm event
that peaked in 1969.
Virtually all significant maxima and minima of SST in the Caribbean Sea correlate
strongly with El Niños and La Niñas respectively (National Weather Service/Climate
Prediction Center 2007). This strong correlation is a good example of atmospheric
teleconnections across the Central American Isthmus. This link is so strong that El
Niños' and La Niñas' effects in the Caribbean Sea have comparable magnitudes with
their counterparts in the Pacific Central-American Coastal LME on the other side of the
Isthmus.
XV Wider Caribbean
659
Figure XV-49.2. Caribbean Sea LME annual mean SST (left) and SST anomalies (right), 1957-2006,
based on Hadley climatology. After Belkin (2008).
Caribbean Sea LME Chlorophyll and Primary Productivity
The Caribbean Sea LME is a Class II, moderate productivity ecosystem (150-300 gCm-
2yr-1)(Figure XV-49.3).
Figure XV-49.3. Caribbean Sea LME trends in chlorophyll a (left) and primary productivity (right), 1998
2006, from satellite ocean colour imagery. Values are colour coded to the right hand ordinate. Figure
courtesy of J. O'Reilly and K. Hyde. Sources discussed p. 15 this volume.
II. Fish and Fisheries
The fisheries of the Caribbean Sea LME are based on a diverse array of resources
(Mahon 2002). Those of greatest importance are spiny lobster (Panulirus argus), queen
conch (Strombus gigas), penaeid shrimps, reef fish, continental shelf demersal fish, deep
slope and bank fish and large coastal pelagics such as king mackerel (Scomberomorus
cavalla), Spanish mackerel (S. maculatus), dolphinfish (Coryphaena hippurus) and
amberjack (Seriola spp.). In addition, fisheries based on stocks of large oceanic fish
such as yellowfin tuna, skipjack tuna, Atlantic blue marlin and swordfish, several of which
have been considered underexploited, have expanded considerably in recent years
(Chakalall & Cochrane 2004). All of the large pelagic stocks are transboundary or Highly
Migratory Species (HMS) and Straddling Stocks (SS), moving in and out of all or most of
the EEZs and extending into the High Seas (Mahon 2003, Die 2004). The distribution of
the large coastal pelagics, which occur largely within the EEZs of Caribbean countries,
660
49. Caribbean Sea LME
also extends into the High Seas (Mahon 2003). The fishery resources are mostly coastal
and intensively exploited by large numbers of small-scale fishers using a variety of gears,
while foreign fleets from distant water fishing nations are known to exploit the region's
High Seas fisheries (Singh-Renton & Mahon 1996). Caribbean countries are often
perceived to be fishing for HMS & SS on the High Seas when they flag foreign vessels on
their open registries (Mahon 2003). This has resulted in problems for several countries of
the Caribbean Community (CARICOM) and there are attempts to eliminate this practice
(FAO 2002). Recreational fishing is an important activity in some of the countries,
particularly for large pelagic fishes (Mahon 2004). Developments in fishing technology,
as well as growing demands for fish have resulted in increasing pressure on the LME's
fish stocks. Additionally, government initiatives have led to substantial increases in
fishing effort, despite the inadequate institutional capacity to manage and monitor the
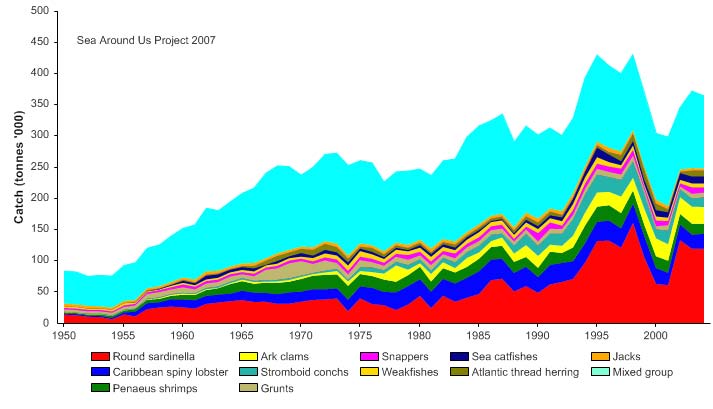
fishing industry. Total reported landings in this LME, which are probably underestimated
(see e.g., contributions in Zeller et al. 2003) showed a general increase to about 430,000
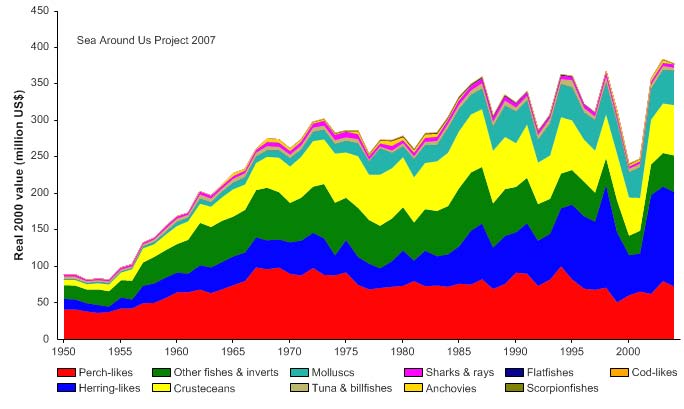
tonnes in the mid-1990s, followed by a slight decline (Figure XV-49.4). In the mid 1990s,
the reported landings were valued at over US$360,000 (in 2000 US dollars; Figure XV-
49.5).
Figure XV-49.4. Total reported landings in the Caribbean Sea LME by species (Sea Around Us 2007).
Figure XV-49.5. Value of reported landings in the Caribbean Sea LME by commercial groups (Sea
Around Us 2007).
XV Wider Caribbean
661
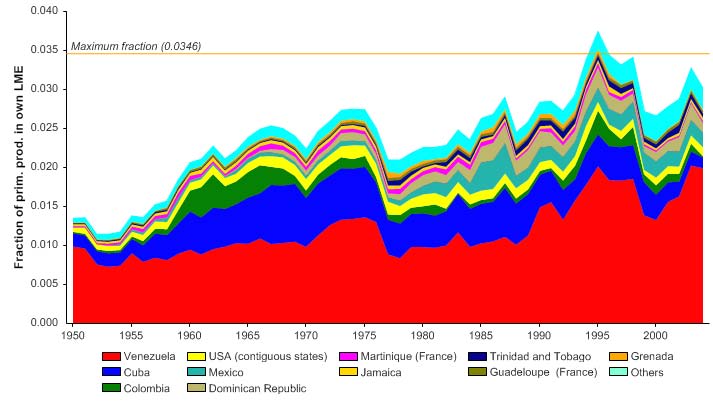
The primary production required (PPR; Pauly & Christensen 1995) to sustain the reported
landings in the LME reached 3% of the observed primary production in 1994 and have
fluctuated between 2.5 to 3% in recent years (Figure XV-49.6). Venezuela accounts for
the largest share of the ecological footprint in this LME.
Figure XV-49.6. Primary production required to support reported landings (i.e., ecological footprint) as
fraction of the observed primary production in the Caribbean Sea LME (Sea Around Us 2007). The
`Maximum fraction' denotes the mean of the 5 highest values.
The decline of the mean trophic level of the reported landings (i.e., the MTI, Pauly &
Watson 2005) is almost linear over the reported period (Figure XV-49.7, top),
representing a classic case of a `fishing down' of the food web in the LME (Pauly et al.
1998). This confirms Pauly & Palomares (2005), who performed a preliminary analysis of
MTI in this region. Indeed, the decline in the mean trophic level would have been greater
were it not for the expansion of the fisheries from the mid 1950 to the mid 1980s as
implied by the increasing FiB index (Figure XV-49.7, bottom).
Figure XV-49.7. Mean trophic level (i.e., Marine Trophic Index) (top) and Fishing-in-Balance Index
(bottom) in the Caribbean Sea LME (Sea Around Us 2007).
662
49. Caribbean Sea LME
The Stock-Catch Status Plots indicate that nearly 80% of the commercially exploited
stocks in the LME are either overexploited or have collapsed (Figure XV-49.8, top) and
these stocks now contribute 60% of the reported landings (Figure XV-49.8, bottom).
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
)
80
%
(
s
30%
u
70
at
st
40%
y
60
b
50%
cks
o
50
f
st
60%
o
40
er
b
70%
m
30
u
N
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 5582)
developing
fully exploited
over-exploited
collapsed
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
80
)
30%
(
%
70
t
us
40%
t
a
60
s
k
c
50%
t
o
50
s
60%
h by
40
t
c
a
70%
C
30
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 5582)
developing
fully exploited
over-exploited
collapsed
Figure XV-49.8. Stock-Catch Status Plots for the Caribbean Sea LME, showing the proportion of
developing (green), fully exploited (yellow), overexploited (orange) and collapsed (purple) fisheries by
number of stocks (top) and by catch biomass (bottom) from 1950 to 2004. Note that (n), the number of
`stocks', i.e., individual landings time series, only include taxonomic entities at species, genus or family
level, i.e., higher and pooled groups have been excluded (see Pauly et al,, this volume, for definitions).
Overexploitation was found to be severe throughout the Caribbean Sea LME (UNEP
2004a, 2004b, 2006). Most coastal resources are considered to be fully or overexploited
and there is increasing evidence that pelagic predator biomass has been depleted
(Mahon 2002, Myers & Worm 2003). Many local fisheries had collapsed by the mid-
1980s following the depletion of lobster, conch and finfish stocks (UNEP 2000).
Overfishing, particularly of herbivorous species, has been identified as a key-controlling
agent on Caribbean reefs leading to shifts in species dominance (Aronson & Precht
2000, Eakin et al. 1997; Hughes, T.P. 1994).
There is concern over the long-term sustainability of spiny lobster stocks due to an
increase in fishing effort for this species. Furthermore, the minimum legal size of lobsters
is well below the size of reproductive maturity in some areas (Richards & Bohnsack
1990). The conch fishery has collapsed in many areas and it is unlikely that conch
catches can be sustained (Richards & Bohnsack 1990, Smith et al. 2000). Several
species of sea turtles are threatened or endangered in many areas as a result of
overexploitation (FAO 1997). Overfishing and reduced abundance of large-sized
carnivorous reef fish such as snappers (Lutjanus spp.) and groupers (Epinephelus spp.)
have been observed in several locations throughout the LME (e.g., Manickchand-
Heileman and Phillip 1999, Charuau et al. 2001, Kramer 2003). Regardless of location,
XV Wider Caribbean
663
legal designation or local fishing regulations, these species have been overexploited in
the entire Western Atlantic region (Ginsberg & Lang 2003). The sustainability of the
groundfish fisheries in the southern Caribbean is also of concern in countries such as
Venezuela and Trinidad and Tobago (Booth et al. 2001). These stocks have experienced
high fishing pressure, particularly from trawlers. In the Gulf of Paria (between Trinidad
and Venezuela), intense pressure from bottom trawling is thought to have contributed to
a reduction in the abundance of species at higher trophic levels and the predominance of
low trophic-level species (Manickchand-Heileman et al. 2004).
There is a clear trend of increasing landings of large pelagic fishes, both coastal and
HMS and SS, by Caribbean countries. This indicates that these fisheries are expanding
steadily, despite the absence of any indication of the levels that may be sustainable
(Mahon 2003). In fact, some of these HMS and SS are already considered to be
overfished, based on assessments carried out by the International Commission for the
Conservation of Atlantic Tunas (ICCAT) (Die 2004). These include the Atlantic swordfish
(ICCAT 2001a) and Atlantic blue marlin and white marlin (ICCAT 2001b). As a result of
both management interventions and high recruitment levels in recent years, the swordfish
stock has been slowly recovering (ICCAT 1999). The Atlantic yellowfin tuna stock is
considered to be fully fished (ICCAT 2001a) but there is concern that the tendency for
fishing effort to increase will ultimately result in overfishing of this species (ICCAT 2001a).
The abundance of Western Atlantic sailfish fell dramatically in the 1960s and has not
increased much since. Current catches seem sustainable, but it is not known how far the
current levels are from maximum sustainable yield (ICCAT 2001b).
The quantity of bycatch and discards in the Caribbean Sea LME is significant, with
bottom trawling for shrimp producing the greatest quantity of bycatch (UNEP/CEP 1996).
Immature individuals of commercially important species generally dominate the shrimp
bycatch. Moreover, the bycatch species composition has changed over the years and
several species have practically disappeared, indicating a dramatic shrinking of their
populations, notably in the case of sharks (Charlier 2001). Considerable quantities of
bycatch, which includes sharks and large coastal pelagics, are also taken in the longline
and other High Seas fisheries (Mahon 2003).
Destructive fishing practices such as dynamite and poison fishing are also contributing to
the decline of some fish species throughout the region (Garzón-Ferreira et al. 2000).
There is a lack of monitoring and enforcement to prevent these illegal practices, except
for coast-watching by communities and coast guards.
Overfishing could have significant transboundary implications in the Caribbean Sea LME.
In addition to the large migratory pelagic fishes, reef organisms, lobster, conch and small
coastal pelagics are also likely to be shared resources by virtue of planktonic larval
dispersal. In many species, larval dispersal lasts for many weeks or months, resulting in
transport across EEZ boundaries (Richards & Bohnsack 1990). Therefore, even these
coastal resources have an important transboundary component to their management.
Therefore, fisheries management should be based on the status of the stock evaluated at
the scale of the entire stock (Die 2004).
III. Pollution and Ecosystem Health
Pollution: Pollution of marine and coastal areas of the Caribbean Sea LME is a major
and recurrent transboundary environmental issue in the region. Land-based pollution
and physical alteration and destruction of habitats are among the major threats to the
coastal and marine environments of the Caribbean Small Island Developing States
(SIDS) (Heileman & Corbin 2006). In addition to land-based sources of pollution, the
discharge of solid waste, wastewater and bilge water from both commercial and cruise
664
49. Caribbean Sea LME
ships as well as other offshore sources are of increasing concern (CAR/RCU 2000,
GEF/CEHI/CARICOM/UNEP 2001). Pollution is moderate in general and severe in some
coastal hotspots particularly around the large cities, especially in the Central
America/Mexico sub-region (UNEP 2004a, 2004b, 2006). The entire Caribbean Sea may
be considered a hotspot in terms of risks from shipping and threats to coral reefs
(Heileman & Corbin 2006).
Sewage is one of the most significant pollutants affecting the coastal environments of the
Wider Caribbean Region (CAR/RCU 2000). Rapid population growth, urbanisation and
the increasing number of ships and recreational vessels have resulted in the discharge of
increasing amounts of poorly treated or untreated sewage into the coastal waters
(CAR/RCU 2000). Of even greater concern are the high bacterial counts that have been
detected in some areas, including in bays where there is a large concentration of boats
and berthing facilities. In addition to microbiological contamination, the input of sewage
contributes high levels of nutrients to coastal areas. This, as well as inputs of fertilisers
from agricultural run-off, have promoted hotspots of eutrophication as well as harmful
algal blooms in some localised areas throughout the region (UNEP 2004a, 2004b). The
estimated nutrient load from land-based sources is 130,000 tonnes nitrogen yr-1 and
58,000 tonnes phosphorus yr-1 (UNEP 2000). Discharges of suspended and dissolved
solids have intensified through human activities, such as deforestation, urbanisation and
agriculture. The region's rivers supply about 300 million tonnes suspended solids per
year to the Greater Caribbean Region (PNUMA 1999). High turbidity and sedimentation
have reduced biodiversity in shallow coastal waters throughout the region (UNEP 2000).
Of growing concern is the increasing amount of solid waste generated within the
Caribbean countries. Because of inadequate collection and disposal facilities, much of
this material eventually ends up on beaches and other coastal areas. About 70-80% of
marine debris originates from the intense shipping traffic, especially cruise ships and oil
tankers that cause an important transboundary movement of marine debris and tar balls
(UNEP 2004a, 2004b). In addition to reducing the aesthetic value of the coastal areas,
solid waste such as plastics are of considerable threat to marine fauna such as turtles,
marine mammals and sea birds.
Chemical contamination from industrial and agricultural activities is severe in some
localised areas (UNEP 2004a, 2004b). For example, pollution by copper, lead and zinc
was found in water and sediments in Cuba, the Dominican Republic and Jamaica
(GEF/UNDP/UNEP 1998). Coastal areas near to oil installations show significant heavy
metal concentrations in sediments, for example, the Santo Domingo coastal zone and
Havana Bay (GEF/UNDP/UNEP 1998, Beltrán et al. 2001). Chemical pollution is severe
in some coastal areas of Central America, which has the highest use of pesticides per
capita and which is expected to increase in the future.
One of the biggest potential threats to the Caribbean Sea LME is that of oil spills.
Because of their petroleum-based industry, countries such as Trinidad, Tobago and
Venezuela continue to have a higher risk of oil spills within their marine environments.
Large volumes of hydrocarbons are discharged from tankers and private vessels in the
region. More than one third of oil spilled at sea between 1983 and 1999 was caused by
accidents at ports and oil installations located in the coastal zone (UNEP 2000).
Thousands of large vessels, including those passing through the Panama Canal,
transport nuclear and other hazardous materials through the Caribbean Sea annually,
which increases the threat of spills of these materials.
Habitat and community modification: The coastal areas of the Caribbean Sea LME
are comprised of habitats such as mangrove wetlands, seagrass beds and coral reefs,
XV Wider Caribbean
665
which dominate the land-sea margin and harbour high biological diversity. These
habitats, however, are being impacted by a range of anthropogenic activities that have
resulted in severe habitat and community modification, particularly around the smaller
islands and along the mainland coast (UNEP 2004a, 2004b, 2006).
Signs of stress are particularly evident in the shallow-water coral reef habitats (Richards
& Bohnsack 1990). Major threats to coral reefs are linked to overexploitation of reef fish
communities, sewage, industrial and agricultural pollution, as well as tourism and
sedimentation (Bryant et al. 1998, Garzón-Ferreira et al. 2000) and global warming.
Recent studies have revealed a trend of serious and continuing long-term decline in the
health of Caribbean coral reefs (Wilkinson 2002, Gardner et al. 2003, Lang 2003,
Wilkinson and Souter 2005). About 30% of Caribbean reefs are now considered to be
either destroyed or at extreme risk from anthropogenic threats (Wilkinson 2000). More
was lost in the 2005 bleaching event (Wilkinson and Souter 2008). Another 20% or more
are expected to be lost over the next 10-30 years if significant action is not taken to
manage and protect them over and beyond existing activities. Dramatic changes in the
community structure of coral reefs have taken place over the past two decades. Prior to
the 1980s, scleractinian (stony) corals dominated Caribbean coral reefs and the
abundance of macroalgae was low. Over the past two decades a combination of
anthropogenic and natural stressors has caused a reduction in the abundance of hard
corals and an increase in macroalgae cover (Richards & Bohnsack 1990, Kramer 2003).
This has been exacerbated by the mass mortality of an important algal grazer, the sea
urchin Diadema sp., in 1983 (Lessios et al. 2001). The worldwide mass coral bleaching
events of 1997-1998 resulting from elevated sea surface temperatures affected coral
reefs in almost the entire Wider Caribbean region (Hoegh-Guldberg 1999), where
bleaching continued until the severe event of 2005. The impact of the bleaching events
varied across the Wider Caribbean, with the Meso-American Barrier Reef sustaining
severe damage.
Hurricanes have also impacted coral reefs in localised areas, for example, in Mexico and
Belize, with varying degree of recovery (Gardner et al. 2005). A range of diseases has
also affected Caribbean coral reefs, starting with black band disease in the early 1970s
followed by white band disease in the late 1970s. Diseases of stony corals and
gorgonians have been reported with increasing frequency (Woodley et al. 2000).
The major threats to the region's mangroves include coastal development and charcoal
production. Many islands have reported deforestation of mangroves for fuel wood, often
by squatters (GEF/CEHI/CARICOM/UNEP 2001). Between 1990 and 2000, 21 out of
26 countries showed decreasing mangrove cover, with annual rates of decline ranging
from 0.3% in the Bahamas to 3.8% in Barbados (FAO 2003). Clearing of mangrove
forests has made the coast more vulnerable to erosion and destroyed the habitat of many
species (UNEP/CEP 1996). Sandy foreshores have also been severely destroyed and
modified due to sand mining and poorly-devised shoreline protection structures (BEST
2002). Seagrass beds in some areas are affected by chronic sedimentation. Habitat
destruction and alteration is significantly impacting the LME's biodiversity. For example,
the population of the West Indian manatee has dramatically declined because of
degradation of essential habitats and because they have been hunted (UNEP/CEP
1995).
Recognising the importance of the Caribbean Sea LME and its resources to economic
development and human well-being, the countries are embarking on numerous
programmes and activities to address the degradation of the marine environment. As a
result, some improvements in the health of this LME are expected in the coming decades
(UNEP 2004a, 2004b).
666
49. Caribbean Sea LME
IV. Socioeconomic Conditions
The Caribbean Sea LME is bordered by 38 countries and dependent territories of the
U.S., France, U.K. and the Netherlands. Sixteen of the independent states and the
14 dependent territories are Small Island Developing States (SIDS). The population of
the Caribbean Sea region is approximately 107 million, with the majority inhabiting the
coastal zones. In addition, each year the population increases considerably due to the
influx of large numbers of tourists during the tourist season. The Caribbean countries,
especially the SIDS, are highly dependent on the marine environment for their economic,
nutritional and cultural well-being. There is a high dependence of the economies of the
islands on tourism, with revenues from tourism ranging between 15 to 99% of total
exports in 90% of the islands (CIA 2005). Marine fisheries also play an important social
and economic role, and are an important source of protein, employment and foreign
exchange earnings in many of the countries.
The socioeconomic impacts of overexploitation vary among the countries, but are
generally slight to moderate (UNEP 2004a, 2004b). The Lesser Antilles Islands suffer
the greatest socioeconomic impacts of overexploitation. Decreasing inshore resources,
increasing harvesting expenses and increasing demand have led to an increase in the
market prices of fish as well as conflicts between traditional and recreational fishers.
Reduced employment opportunities in the fisheries sector have forced fishers to seek
other sources of income. Declining fisheries resources also threaten the food security of
fishers and others who are dependent on fisheries resources.
The socioeconomic impacts of pollution are moderate to severe, particularly in the Lesser
Antilles and the Central American countries (UNEP 2004a, 2004b). Human health is
threatened and the propagation of disease vectors promoted by the discharge of non-
treated sewage and other contaminants (UNEP 2000). Where algal biomasses are
significantly elevated due to eutrophication, such as in nutrient/sewage-enriched areas,
the risk of disease and ciguatera poisoning is high (PNUMA 1999). Pollution has also
diminished the aesthetic value of some parts of the region resulting in a loss of revenue
from tourism (UNEP/CEP 1997).
The socioeconomic impacts of habitat modification range from slight to severe (UNEP
2004a, 2004b). The Caribbean islands are particularly affected by habitat degradation,
as are the Central American countries. The impacts include medium to long-term loss of
employment and income opportunities in the tourism sector, loss of recreational, cultural,
educational, scientific values as well as costs of restoration of modified ecosystems
(UNEP 2004a, 2004b). Habitats, such as mangroves and coral reefs, perform an
important role in coastal protection and stabilisation. Therefore, the destruction of these
coastal habitats has serious implications for the Caribbean Sea countries, particularly the
SIDS, in view of rising sea levels and an increase in the frequency and intensity of storms
and hurricanes (UNEP 2005).
V. Governance
With 38 countries and dependencies in the LME, the EEZs form a complete mosaic,
resulting in many transboundary resource management issues, even at relatively small
spatial scales. The need for countries of the Wider Caribbean to pay attention to the
management of transboundary marine resources is well documented (Mahon 1987, FAO
1997). The fisheries initiatives in the region are partly governed by international
frameworks such as UNCLOS, the UN Fish Stocks Agreement and the FAO Code of
Conduct for Responsible Fisheries. At the regional level, there are several initiatives for
the coordination of fisheries management (Mahon 2003). These are broad in scope,
XV Wider Caribbean
667
covering resources that range in distribution from coastal/national to HMS & SS. Among
them are the FAO Western Central Atlantic Fisheries Commission, the Latin American
Organisation for Fishery Development, CARICOM Regional Fisheries Mechanism, the
Caribbean Fisheries Management Council and the Intergovernmental Oceanic
Commission Sub-commission for the Caribbean (IOCARIBE). Operating at the
international level are ICCAT and the International Whaling Commission. In 2001, the
UN Fish Stocks Agreement that seeks to implement the provisions of UNCLOS related to
conservation and management of HMS & SS came into force.
Despite a recognised need, there is no Regional Fisheries Management Organisation for
the Wider Caribbean, including the Caribbean Sea LME, with a mandate to manage the
fisheries resources. The most established and operational fisheries management
organisation with relevance to the Caribbean Sea LME is ICCAT, which has the mandate
to manage all tuna and tuna-like species in the Atlantic. The coastal species are
perceived as being western Atlantic stocks that could be managed by the countries of the
Wider Caribbean, whereas the oceanic stocks require a level of collaboration that would
be best facilitated by an organisation such as ICCAT (Mahon 2003).
Regional programmes related to the marine environment include the UNEP's Regional
Seas Programme, the Caribbean Coastal Marine Productivity Programme and the
Caribbean Environment Programme (CEP), a sub-programme of UNEP's Regional Seas
Programme. The aim of CEP is to promote regional cooperation for the protection and
development of the marine environment of the Wider Caribbean Region. CEP, which is
facilitated by the Caribbean Regional Coordinating Unit located in Jamaica, is involved in
several regional projects and initiatives including the International Coral Reef Initiative
and its Action Network.
A number of marine environmental policy frameworks have been developed in the
Caribbean. These include the 1981 CEP Caribbean Action Plan and the Convention for
the Protection and Development of the Marine Environment in the Wider Caribbean
Region (the Cartagena Convention) and its three protocols (Protocol Concerning
Cooperation in Combating Oil Spills in the Wider Caribbean Region, Protocol Concerning
Specially Protected Areas and Wildlife in the Wider Caribbean Region, and Protocol
Concerning Marine Pollution from Land-Based Sources and Activities). In 1991, the
Marine Environment Protection Committee of the International Maritime Organisation
designated the Gulf of Mexico and the Wider Caribbean Region as a Special Area under
Annex V of the MARPOL Convention. An ongoing initiative to have the Caribbean Sea
internationally recognised as a special area in the context of sustainable development led
to the adoption in 2003 by the UN General Assembly of the resolution `Promoting an
integrated management approach to the Caribbean Sea area in the context of
sustainable development'.
GEF is supporting the project `Integrating Watershed and Coastal Area Management in
Small Island Developing States of the Caribbean'. The overall objective of this project is
to assist participating countries in improving their watershed and coastal zone
management practices. The project `Sustainable Management of the Shared Living
Marine Resources of the Caribbean Large Marine Ecosystem and Adjacent Regions' has
been developed by IOCARIBE and is being implemented. The goal of this project is the
sustainable management of the shared living marine resources of the LME and adjacent
areas through an integrated management approach. The project is focused on aligning
institutions on the national and regional scales to sustainably manage near shore and
deep-water fisheries and related habitats of the LME, including the development and use
of a knowledge base to support institutional decision-making. One of the objectives of
this project is the preparation of a Transboundary Diagnostic analysis (TDA) and
Strategic Action Plan (SAP) for the Caribbean Sea LME and Adjacent Regions.
668
49. Caribbean Sea LME
References
Aronson, R.B. and Precht, W.F. (2000). Herbivory and algal dynamics on the coral reef at
Discovery Bay, Jamaica. Limnology and Oceanography 45:251255.
Belkin, I.M. (2008) Rapid warming of Large Marine Ecosystems, Progress in Oceanography, in
press.
Belkin, I.M., Cornillon, P.C., and Sherman, K. (2008). Fronts in Large Marine Ecosystems of the
world's oceans. Progress in Oceanography, in press.
Beltrán, J., Martín, A., Ruiz, F., Regadera, R., Mancebo, H. and Ramírez, M. (2001). Control y
Evolución de la Calidad Ambiental de la Bahía de La Habana y el Litoral Adyacente. Informe
Final Vigilancia Ambiental para la Bahía de La Habana. Centro de Ingeniería y Manejo
Ambiental de Bahías y Costas (Cimab), Cuba.
BEST (2002). Bahamas Environmental Handbook. Bahamas Environment Science and Technology
Commission. The Government of the Bahamas. Nassau, New Providence.
Booth, A., Charuau, A., Cochrane, K., Die, D., Hackett, A., Lárez, A., Maison, D., Marcano, L.A.,
Phillips, T., Soomai, S., Souza, R., Wiggins, S. and IJspol, M. (2001). Regional Assessment of
the Brazil-Guianas Groundfish Fisheries. FAO Fisheries Report 651: 22-36.
Bryant, D., Burke, L., McManus, J.W. and Spalding, M. (1998). Reefs at Risk: A Map-based
Indicator of Threats to the World's Coral Reefs. World Resource Institute, Washington, D.C.,
U.S.
CAR/RCU (2000). An Overview of Land Based Sources of Marine Pollution. Caribbean
Environment Programme/Regional Coordinating Unit, Kingston, Jamaica. www.cep.unep.org
/issues/lbsp.html
Chakalall, B. and Cochrane, K. (2004). Issues in the management of large pelagic fisheries in
CARICOM countries, p 1-4 in: Mahon, R. and McConney, P. (eds), Management of Large
Pelagic Fisheries in CARICOM Countries. FAO Fisheries Technical Paper 464.
Charlier, P. (2001). Review of environmental considerations in management of the Brazil-Guianas
shrimp and groundfish fisheries. FAO Fisheries Report 651: 37-57.
Charuau, A., Cochrane, K., Die, D., Lárez, A., Marcano, L., Phillips, T., Soomai, S., Souza, R.,
Wiggins, S. and IJspol, M. (2001). Regional assessment of red snapper, Lutjanus purpureus.
FAO Fisheries Report 651: 15-21.
CIA (2005). World Factbook. www.cia.gov/cia/publications/factbook/
Die, D. (2004). Status and assessment of large pelagic resources, p 15-44 in: Mahon, R. and
McConney, P. (eds), Management of Large Pelagic Fisheries in CARICOM Countries. FAO
Fisheries Technical Paper 464.
Eakin, C.M., McManus, J.W., Spalding, M.D. and Jameson, S.C. (1997). Coral reef status around
the world: Where are we and where do we go from here? p 277-282 in: Lessios, H.A. and
MacIntyre, I.G., (eds), Proceedings of the 8th International Coral Reef Symposium, Smithsonian
Tropical Research Institute, Republic of Panama.
FAO (1997). Western Central Atlantic Fishery Commission Report of the Seventh Session of the
Working Party on Marine Fishery Resources, Belize City, Belize 2-5 December 1997. FAO
Fisheries Report 576.
FAO (2002). Preparation for Expansion of Domestic Fisheries for Large Pelagic Species by
CARICOM Countries. Large Pelagic Fisheries in CARICOM Countries: Assessment of the
Fisheries and Options for Management. FI: TCP/RLA/0070 Field Document 1.
FAO (2003). Report of the Fourth Session of the Advisory Committee on Fisheries Research
(ACFR). FAO Fisheries Report 699 (FIPL/R699).
Gardner, T.A., Côté, I.M., Gill, J.A., Grant, A. and Watkinson, A.R. (2003). Long-term region-wide
declines in Caribbean corals. Science 301: 958-960.
Gardner, T. A., I. M. Cote, J. A. Gill, A. Grant, and A. R. Watkinson. 2005. Hurricanes and
Caribbean coral reefs: Impacts, recovery patterns, and role in long-term decline. Ecology
86:174-184.
Garzón-Ferreira, J., Cortés, J., Croquer, A., Guzmán, H., Leao, Z. and Rodríguez-Ramírez, A.
(2000). Status of Coral reefs in Southern Tropical America: Brazil, Colombia, Costa Rica,
Panamá and Venezuela, p 331-348 in: Wilkinson, C. (ed), Status of coral reefs of the world:
2000. Australian Institute of Marine Sciences, Townsville, Queensland, Australia.
XV Wider Caribbean
669
GEF/CEHI/CARICOM/UNEP (2001). Integrating Watershed and Coastal Area Management in
Small Island Developing States of the Caribbean. At www.cep.unep.org/programmes/
amep/GEF-IWCAM/GEF-IWCAM.htm
GEF/UNDP/UNEP (1998). Planning and Management of Heavily Contaminated Bays and Coastal
Areas in The Wider Caribbean. Regional Project RLA/93/G41. Final Report. Havana, Cuba.
Ginsberg, R.N. and Lang, J.C. (2003). Foreword, p xvxiv in: Lang, J. C. (ed), Status of Coral
Reefs in the Western Atlantic. Results of Initial Surveys, Atlantic and Gulf Rapid Reef
Assessment (AGRRA) Programme. Atoll Research Bulletin 496. National Museum of Natural
History, Smithsonian Institute, Washington, D.C., U.S.
Heileman, S. and Corbin, C. (2006). Caribbean SIDS, p. 213 245 in: UNEP/GPA (2006), The
State of the Marine Environment: Regional Assessments. UNEP/GPA, The Hague.
Heyman, W. D. and Kjerfve, B. (1999) Hydrological and oceanographic considerations for
integrated coastal zone management in southern Belize, Environmental Management 24(2):
229-245.
Hoegh-Guldberg, O. (1999). Climate Change, Coral Bleaching and the Future of the World's Coral
Reefs. Greenpeace International, Sydney, New South Wales, Australia.
Hughes, T.P. (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral
reef. Science 265:1547-1551.
ICCAT (1999). International Commission for the Conservation of Atlantic Tuna. Report for Biennial
Period, 19981999, Part 1. Madrid, Spain.
ICCAT (2001a). International Commission for the Conservation of Atlantic Tuna. Report for Biennial
Period, 20002001, Part 1. Madrid, Spain.
ICCAT (2001b). International Commission for the Conservation of Atlantic Tuna. Report of the
Fourth ICCAT Billfish Workshop. ICCAT Collective Volume of Scientific Papers 53:1-130.
Kramer, P. (2003). Synthesis of coral reef health indicators for the Western Atlantic: Results of the
AGGRA Programme (1997-2000), p 1-57 in Lang J.C. (ed), Status of Coral Reefs in the
Western Atlantic: Results of Initial Surveys, Atlantic and Gulf Rapid Reef Assessment (AGGRA)
Programme. Atoll Research Bulletin 496. National Museum of Natural History, Smithsonian
Institute, Washington, DC, U.S.
Lang, J. C. (2003). Caveats for the AGGRA `Initial Results' Volume, p xvxx in: Lang, J. C. (ed),
Status of Coral Reefs in the Western Atlantic. Results of Initial Surveys, Atlantic and Gulf Rapid
Reef Assessment (AGRRA) Programme. Atoll Research Bulletin 496.
Lessios, H.A., Garrido, M.J. and Kessing, B.D. (2001). Demographic history of Diadema antillarum,
a keystone herbivore on Caribbean reefs. Proceedings of the Royal Society of London, Series
B Biological Sciences 22; 268(1483):2347-2353.
Mahon, R. (2002). Living Aquatic Resource Management, p 143-218 in: Goodbody, I. and Thomas-
Hope, E. (eds), Natural Resource Management for Sustainable Development in the Caribbean.
Canoe Press, UWI, Kingston, Jamaica.
Mahon, R. (2003). Implementation of the Provisions of the UN Fish Stocks Agreement: Conditions
for Success The Case of the Wider Caribbean. IDDRA, Portsmouth, U.K.
Mahon, R. (2004). Harvest Sector, p 45-78, in: Mahon, R. and McConney, P. (eds), Management of
Large Pelagic Fisheries in CARICOM Countries. FAO Fisheries Technical Paper 464.
Mahon, R., ed. (1987). Report and Proceedings of the Expert Consultation on Shared Fishery
Resources of the Lesser Antilles Region. FAO Fisheries Report 383.
Manickchand-Heileman, S. and Phillip, D.A.T. (1999). Contribution to the biology of the vermilion
snapper Rhomboplites aurorubens in Trinidad and Tobago, West Indies. Environmental Biology
of Fishes 55:413-421.
Manickchand-Heileman, S., Mendoza, J., Lum Kong, A. and Arocha, F. (2004). A trophic model for
exploring possible ecosystem impacts of fishing in the Gulf of Paria, between Venezuela and
Trinidad. Ecological Modelling 172:307-322.
Müller-Karger, F.E. (1993). River Discharge Variability Including Satellite-observed Plume-dispersal
Patterns, p 162-192 in: Maul, G. (ed), Climate Change in the Intra-Americas Sea. United
Nations Environmental Programme (Wider Caribbean Region) and Intergovernmental
Oceanographic Commissions (Caribbean and Adjacent Regions).
Myers, R.A. and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature
423:280-283.
National Weather Service/Climate Prediction Center (2007) Cold and warm episodes by seasons [3
month running mean of SST anomalies in the Niño 3.4 region (5oN-5oS, 120o-170oW)][based on
the 1971-2000 base period], www.cpc.ncep.noaa.gov/products/analysis_monitoring/
ensostuff/ensoyears.shtml
670
49. Caribbean Sea LME
Pauly, D. and Christensen, V. (1995). Primary production required to sustain global fisheries.
Nature 374: 255-257.
Pauly, D. and Palomares, M.L. (2005). Fishing down marine food webs: it is far more pervasive
than we thought. Bulletin of Marine Science 76(2): 197-211.
Pauly, D. and Watson, R. (2005). Background and interpretation of the `Marine Trophic Index' as a
measure of biodiversity. Philosophical Transactions of the Royal Society: Biological Sciences
360: 415-423.
Pauly, D., Christensen, V., Dalsgaard, J., Froese R. and Torres, F.C. Jr. (1998). Fishing Down
Marine Food Webs. Science 279: 860-863.
PNUMA (1999). Evaluación sobre las Fuentes Terrestres y Actividades que Afectan al Medio
Marino, Costero y de Aguas Dulces Asociadas en la Región del Gran Caribe. Informes y
Estudios del Programa de Mares Regionales del PNUMA 172. Oficina de Coordinación del
PAM, Programa Ambiental del Caribe.
Richards, W.J. and Bohnsack, J.A. (1990). The Caribbean Sea: A Large Marine Ecosystem in
crisis, p 44-53 in: Sherman, K., Alexander, L.M. and Gold, B.D. (eds), Large Marine
Ecosystems: Patterns, Processes and Yields. AAAS, Washington D.C., U.S.
Sea Around Us (2007). A Global Database on Marine Fisheries and Ecosystems. Fisheries Centre,
University British Columbia, Vancouver, Canada. www.seaaroundus.org/lme/
SummaryInfo.aspx?LME=12
Singh-Renton, S. and Mahon, R. (1996). Catch, Effort and CPUE Trends for Offshore Pelagic
Fisheries in and Adjacent to the Exclusive Economic Zones (EEZs) of Several CARICOM
States. CARICOM Fishery Report 1.
Smith, A.H., Archibald, M., Bailey, T., Bouchon, C., Brathwaite, A., Comacho, R., Goerge, S.,
Guiste, H., Hasting, M., James, P., Jeffrey-Appleton, C., De Meyer, K., Miller, A., Nurse, L.,
Petrovic, C. and Phillip, P. (2000). Status of the Coral Reefs in the Eastern Caribbean: The
OECS, Trinidad and Tobago, Barbados, the Netherlands Antilles and the French Caribbean, in:
Wilkinson, C. (ed), Status of Coral Reefs of the World 2000. Australian Institute of Marine
Science, Australia.
Spalding, M., Ravilious, C. and Green, E.P. (2001). World Atlas of Coral Reefs. United Nations
Environment Programme World Conservation Monitoring Centre, Cambridge, U.K.
UNEP (2000). Global Environment Outlook 2000. Earthscan Publications Ltd., London.
UNEP (2004a). Bernal, M.C., Londoño, L.M., Troncoso, W., Sierra- Correa, P.C. and Arias-Isaza,
F.A. Caribbean Sea/Small Islands, GIWA Regional Assessment 3a. University of Kalmar,
Kalmar, Sweden. http://www.giwa.net/publications/r3a.phtml
UNEP (2004b). Villasol, A. and Beltrán, J. Caribbean Islands, GIWA Regional Assessement 4.
Fortnam, M. and Blime P. (eds), University of Kalmar, Kalmar, Sweden.
http://www.giwa.net/publications/r4.phtml
UNEP (2005). Caribbean Environment Outlook. UNEP, Nairobi, Kenya.
UNEP (2006). Isaza, C.F.A., Sierra-Correa, P.C., Bernal-Velasquez, M., Londoño, L.M. and W.
Troncoso. Caribbean Sea/Colombia & Venezuela, Caribbean Sea/Central America & Mexico,
GIWA Regional assessment 3b, 3c. University of Kalmar, Kalmar, Sweden.
http://www.giwa.net/publications/r3bc.phtml
UNEP/CEP (1995). Regional Management Plan for the West Indian Manatee, Trichechus manatus.
CEP Technical Report 35. UNEP Caribbean Environment Programme, Kingston, Jamaica.
UNEP/CEP (1996). Directrices y Criterios Comunes para las Áreas Protegidas en la Región del
Gran Caribe. Identificación, Selección, Establecimiento y Gestión. Informe Técnico del PAC 37.
UNEP Caribbean Environment Programme, Kingston, Jamaica.
UNEP/CEP (1997). Coastal Tourism in the Wider Caribbean Region: Impacts and Best
Management Practices. CEP Technical Report 38, UNEP Caribbean Environment Programme,
Kingston, Jamaica.
Wilkinson, C., ed. (2000). Status of Coral Reefs of the World 2000. Australian Institute of Marine
Science, Australia.
Wilkinson, C., ed. (2002). Status of Coral Reefs of the World 2002. Australian Institute of Marine
Sciences, Townsville, Australia.
Wilkinson, C. L., and Souter, D.. (eds) 2008. Status of Caribbean coral reefs after bleaching and
hurricanes in 2005 Global Coral Reef Monitoring Network, and Reef and Rainforest Research
Centre, Townsville. Available at: http://www.coris.noaa.gov/activities/caribbean_rpt/
Wilkinson, C.L. and Souter, D. (eds) 2008. Status of Caribbean coral reefs after bleaching and
hurricanes in 2005 Global Coral Reef Monitoring Network, and Reef and Rainforest Research
Centre, Townsville.
XV Wider Caribbean
671
Woodley, J., Alcolado, P., Austin, T., Barnes, J., Claro-Madruga, R., Ebanks-Petrie, G., Estrada,
R., Geraldes, F., Glasspool, A., Homer, F., Luckhurst, B., Phillips, E., Shim, D., Smith, R.,
Sullivan-Sealy, K., Vega, M., Ward, J. and Wiener, J. (2000). Status of Coral Reefs in the
Northern Caribbean and Western Atlantic. In: Wilkinson, C. (ed), Status of Coral Reefs of the
World 2000. Australian Institute of Marine Science, Townsville, Australia.
Zeller D., Booth, S., Mohammed, E. and Pauly, D. (eds). (2003). From Mexico to Brazil: Central
Atlantic fisheries catch trends and ecosystem models. Fisheries Centre Research Reports
11(6), 264 p.
672
49. Caribbean Sea LME
XV Wider Caribbean
673
XV-50 Gulf of Mexico LME
S. Heileman and N. Rabalais
The Gulf of Mexico LME is a deep marginal sea bordered by Cuba, Mexico and the U.S.
It is the largest semi-enclosed coastal sea of the western Atlantic, encompassing more
than 1.5 million km2, of which 1.57% is protected, as well as 0.49% of the world's coral
reefs and 0.02% of the world's sea mounts (Sea Around Us 2007). The continental shelf
is very extensive, comprising about 30% of the total area and is topographically very
diverse. Oceanic water enters this LME from the Yucatan channel and exits through the
Straits of Florida creating the Loop Current, a major oceanographic feature and part of
the Gulf Stream System (Lohrenz et al. 1999). The LME is strongly influenced by
freshwater input from rivers, particularly the Mississippi-Atchafalaya, which accounts for
about two-thirds of the flows into the Gulf (Richards & McGowan 1989). Forty-seven
major estuaries are found in this LME (Sea Around Us 2007). Important hydrocarbon
seeps exist in the southernmost and northern parts of the LME (Richards & McGowan
1989). A major climatological feature is tropical storm activity, including hurricanes.
Book chapters pertaining to this LME are by Richards & McGowan (1989), Brown et al.
(1991). A volume on this LME is edited by Kumpf et al. (1999).
I. Productivity
The Gulf of Mexico LME is a moderately high productivity ecosystem (<300 gCm-2/yr-1).
Conditions range from eutrophic in the coastal waters to oligotrophic in the deeper ocean.
Lohrenz et al. (1999) distinguished among local scale, mesoscale and synoptic scale
processes that influence primary productivity in the LME. Upwelling along the edge of
the Loop Current as well as its associated rings and eddies are major sources of
nutrients to the euphotic zone. It has been suggested that this upwelling causes a 2- to
3-fold increase in the annual rate of primary production in the Gulf (Wiseman & Sturges
1999). The region of the Mississippi River outflow has the highest measured rates of
primary production (Lohrenz et al. 1990). The Gulf's primary productivity supports an
important global reservoir of biodiversity and biomass of fish, sea birds and marine
mammals. Each summer, widespread areas on the northern continental shelf are
affected by severe and persistent hypoxia (Rabalais et al. 1999a).
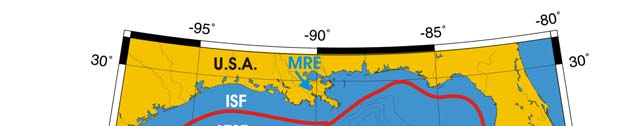
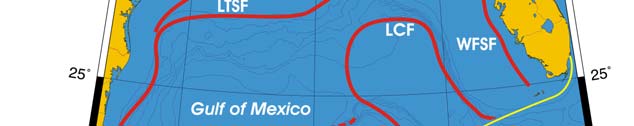
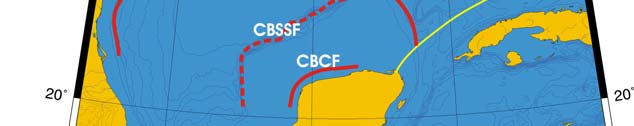
Oceanic Fronts (Belkin et al. 2008)(Figure XV-50.1): From December through March,
two major fronts emerge over two shelf areas, the West Florida Shelf (WFS) and
Louisiana-Texas Shelf (LTS). The WFS Front (WFSF) extends over the mid-shelf,
whereas the LTS Front (LTSF) is located closer to the shelf break. Both fronts form
owing to cold air outbreaks (e.g., Huh et al. 1978). Huge freshwater discharge from the
Mississippi River Estuary (MRE) and rivers of the Florida Panhandle contributes to the
fronts' development and maintenance. Compared to these northern fronts, the
Campeche Bank Shelf-Slope Front (CBSSF) and Campeche Bank Coastal Front (CBCF)
in the south are weak and unstable. The Loop Current Front (LCF) is always present at
the inshore boundary of the namesake front, best defined in winter.
674
50. Gulf of Mexico LME
Figure XV-50.1. Fronts of the Gulf of Mexico. Acronyms: CBCF, Campeche Bank Coastal Front;
CBSSF, Campeche Bank Shelf-Slope Front (most probable location); ISF, Inner Shelf Front; LCF, Loop
Current Front; LTSF, Louisiana-Texas Shelf Front; MRE, Mississippi River Estuary; WFSF, West
Florida Shelf Front. Yellow line, LME boundary. After Belkin et al. (2008).
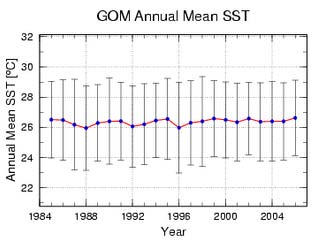
Gulf of Mexico LME SST (Belkin 2008)(Figure XV-50.2):
Linear SST trend since 1957: 0.19°C.
Linear SST trend since 1982: 0.31°C.
The Gulf of Mexico thermal history is quite peculiar. The global cooling of the 1960s
transpired here as an SST drop of <1°C, followed by a slow warming until present. The
relatively slow warming of the last 50 years was modulated by strong interannual
variability with a typical magnitude of 0.5°C. The all-time record high of 26.4°C in 1972
was a major event since SST increased by nearly 0.8°C in just two years. This event
was probably localized with the Gulf of Mexico. An alternative explanation involves a
gradual drift of a record-strong positive SST anomaly of 1969 from the Caribbean Sea
LME. The time lag of three years between the Caribbean Sea SST maximum and the
Gulf of Mexico SST maximum makes this correlation tenuous.
The relatively slow warming, if any, of the Gulf of Mexico is also evident from satellite
SST data from 1984-2006 assembled and processed at NOAA/AOML (Figure XV-50.2a).
Even though the annual mean SST change little since 1957, summer SST in the Atlantic
tropical areas rose substantially since the 1980s, which is thought to have resulted in a
recent increase of destructiveness of tropical cyclones, including those that hit the Gulf of
Mexico (Emanuel 2005).
XV Wider Caribbean
675
a
b
Figure XV-50.2a. Time series of annual mean SST in the Gulf of Mexico derived from satellite data,
1984-2006, processed at NOAA's Atlantic Oceanographic and Meteorological Laboratory, Miami,
Florida. Source: www.aoml.noaa.gov/phod/regsatprod/gom/sst_anm.php. Figure XV-50.2b. Gulf of
Mexico LME annual mean SST (left) and SST anomalies (right), 1957-2006, based on Hadley climatology.
After Belkin (2008).
Gulf of Mexico LME Chlorophyll and Primary Productivity: The Gulf of Mexico LME
is a Class II, moderately-high productivity ecosystem (150-300 gCm-2/yr-1)(Figure XV-
50.3).
Figure XV-50.3. Gulf of Mexico LME trends in chlorophyll a (left) and primary productivity (right), 1998-
2006, from satellite ocean colour imagery Values are colour coded to the right hand ordinate. Figure
courtesy of J. O'Reilly and K. Hyde. Sources discussed p. 15 this volume.
676
50. Gulf of Mexico LME
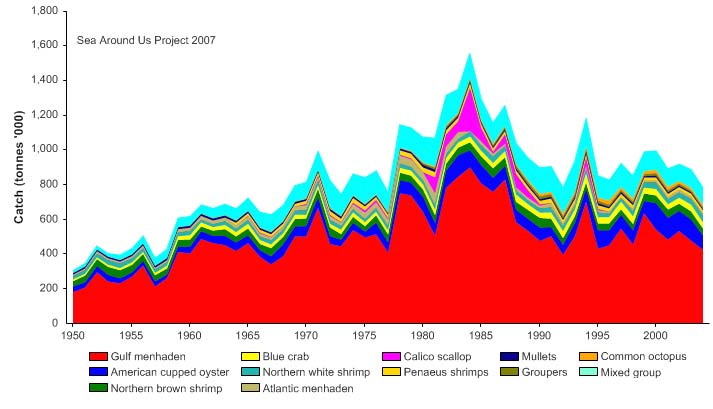
II. Fish and Fisheries
The Gulf of Mexico LME fisheries are multispecies, multigear and multifleet in character
and include artisanal, commercial and recreational fishing. Species of economic
importance include brown shrimp (Farfantepenaeus aztecus), white shrimp (Litopenaeus
setiferus), pink shrimp (Farfantepenaeus duorarum), Gulf menhaden (Brevoortia
patronus), king mackerel (Scomberomorus cavalla), Spanish mackerel (S. maculatus),
red grouper (Epinephelus morio), red snapper (Lutjanus campechanus), seatrout, tuna
and billfish (NOAA/NMFS 1999). Reported landings from this LME are dominated by
herrings, sardines and anchovies (FAO 2003), but they underestimate total catches, due
to non-inclusion of much of the discarded fish bycatch of shrimp trawlers (see e.g.
contributions in Yañez-Arancibia, 1985). Total reported landings increased to over
1.5 million tonnes in 1984, and then declined to 780,000 tonnes in 2004 (Figure XV-50.4).
Between 1969 and 1999, the annual value of the reported landings has been over US$1
billion (in 2000 US dollars) and reached US$2 billion in 1979 (Figure XV-50.5).
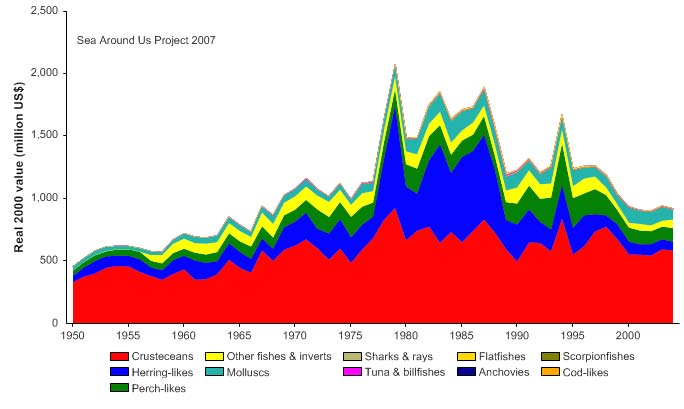
Figure XV-50.4. Total reported landings in the Gulf of Mexico LME by species (Sea Around Us 2007).
Figure XV-50.5. Value of reported landings in the Gulf of Mexico LME by commercial groups (Sea
Around Us 2007).
XV Wider Caribbean
677
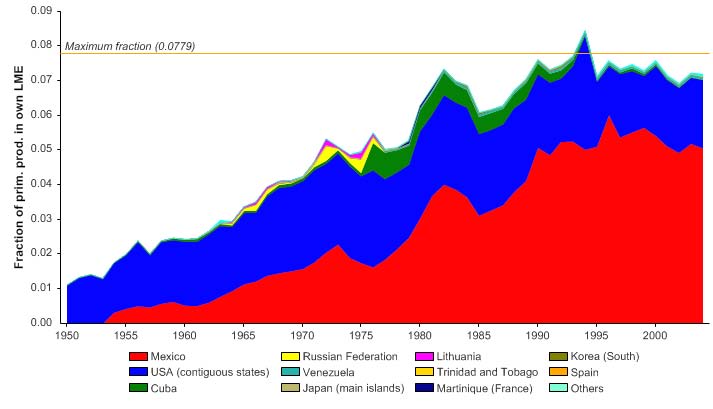
The primary production required (PPR; Pauly & Christensen 1995) to sustain the reported
landings in the LME reached 8% of the observed primary production in 1994 (Figure XV-
50.6), but this PPR underestimate due to the high level of shrimp bycatch not included in
the underlying statistics. Mexico and the USA account for the majority of the ecological
footprints in this LME.
Figure XV-50.6. Primary production required to support reported landings (i.e., ecological footprint) as
fraction of the observed primary production in the Gulf of Mexico LME (Sea Around Us 2007). The
`Maximum fraction' denotes the mean of the 5 highest values.
The mean trophic level of the reported landings (i.e., the MTI; Pauly & Watson 2005) has
increased slightly from the early 1950s to 2004 (Figure XV-50.7, top). The very low value
of MTI (2.3-2.5) is due to the high proportion of small low trophic pelagic fishes,
especially Gulf menhaden and shrimps in the landings, and the exclusion of the shrimp
trawler bycatches in valuation of the mean trophic level.
Figure XV-50.7. Mean trophic level (i.e., Marine Trophic Index) (top) and Fishing-in-Balance Index
(bottom) in the Gulf of Mexico LME (Sea Around Us 2007).
678
50. Gulf of Mexico LME
As for the observed increase in MTI, this may also be an artefact, as can be inferred from
the work of Baisre (2000). He found, based, on detailed catch data from Cuba that
included bycatch and covered an extended period (1935-1995), that a `fishing down' of
food webs (Pauly et al. 1998) is occurring in the region. The decline of the FiB index
from the mid 1980s (Figure XV-50.7, bottom) is likely a result of the diminished reported
landings.
The Stock-Catch Status Plots indicate that collapsed and overexploited stocks now
account for over 70% of all commercially exploited stocks in the LME (Figure XV-50.8,
top), with overexploited stocks contributing 60% of the reported landings (Figure XV-50.8,
bottom).
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
)
80
%
(
s
30%
u
70
at
st
40%
y
60
b
ks
50%
c
50
t
o
f
s
60%
40
r
o
e
70%
mb
30
u
N
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 4739)
developing
fully exploited
over-exploited
collapsed
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
80
)
%
30%
(
70
s
u
at
40%
60
st
ck
50%
t
o
50
s
y
60%
b
h
40
t
c
a
70%
C
30
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 4739)
developing
fully exploited
over-exploited
collapsed
Figure XV-50.8. Stock-Catch Status Plots in the Gulf of Mexico LME, showing the proportion of
developing (green), fully exploited (yellow), overexploited (orange) and collapsed (purple) fisheries by
number of stocks (top) and by catch biomass (bottom) from 1950 to 2004. Note that (n), the number of
`stocks', i.e., individual landings time series, only include taxonomic entities at species, genus or family
level, i.e., higher and pooled groups have been excluded (see Pauly et al, this volume for definitions).
Overexploitation was found to be moderate as a whole, but severe on the Campeche
Bank in the southwestern Gulf. Intensive fishing is the primary force driving biomass
changes in the LME, with climatic variability the secondary driving force (Sherman 2003).
In general, the fish stocks of this LME are impacted by excessive recreational and
commercial fishing pressure (Birkett & Rapport 1999). Both the traditional and the more
recent fisheries have reached their harvesting limits and several species are
overexploited (Arreguín-Sánchez et al. 1999, Brown et al. 1991, NOAA/NMFS 1999,
Shipp 1999). Spanish mackerel, shark and coastal pelagics showed severe declines
under intense fishing pressure during the late 1980s (Shipp 1999). Other commercially
important species that have been overexploited are the red drum and spotted seatrout,
and there has been concern over the sustainability of the fishery for amberjack and gag
grouper (Shipp 1999). The Gulf menhaden stocks fluctuated and then declined under
XV Wider Caribbean
679
heavy fishing pressure and other stresses (Birkett & Rapport 1999). Several stocks of
reef fish, including red, Nassau and goliath groupers, are also overexploited
(NOAA/NMFS 1999). The red snapper is considered the most severely overexploited
species in the Gulf, and its recovery is deterred by the high mortality of its juveniles in
shrimp trawl bycatch. Stocks of large migratory pelagic fish are also under threat from
overfishing. Landings of bluefin tuna dropped precipitously in the late 1970s and the
stocks are also considered to be severely overexploited. Likewise, other large pelagics
such as swordfish and blue and white marlin are also thought to be overexploited.
In the early 1980s, the shrimp fishery on the continental shelf off Campeche in the
southwestern Gulf of Mexico LME formed the base of the economy in this area (Arreguín-
Sánchez et al. 2004). This fishery, particularly for the pink shrimp has collapsed, with
annual harvests falling from 27,000 tonnes in the early 1970s to 3,000 tonnes or less
(Arreguín-Sánchez et al. 1997). There has been evidence of marked declines in the
abundance of pink and white shrimps in this area as a result of heavy fishing on juveniles
inshore as well as on spawners in offshore areas (Gracia & Vasquez-Bader 1999). Also
in this area, the red grouper and the brackish water clam fisheries collapsed in the late
1980s (Arreguín-Sánchez et al. 1999). As a result of these declines, the fisheries in the
Campeche area focus on other, less valuable species, such as finfish and octopus
(Arreguín-Sánchez et al. 2004).
Many of these fisheries are now under management (e.g., seasonal closures, size limits,
quotas) and some have started to show recovery (Arreguín-Sánchez et al. 1999, Brown
et al. 1991, NOAA/NMFS 1999, Shipp 1999). For example, Spanish mackerel, Gulf
menhaden as well as white, pink and brown shrimps are now considered to be either in a
state of recovery or at least are no longer overexploited. However, concern still exists
over continued overcapitalisation and the shift of fishing to lower tropic levels and smaller
sizes of fish, which are the prey of species supporting valuable, fully developed fisheries
(Brown et al. 1991, UNDP/GEF 2004). Harvest of prey species may therefore have long-
term negative impacts on the production of currently harvested species in the Gulf and
should be accompanied by research into important ecological relationships and
multispecies effects (Brown et al. 1991, Pauly et al. 1999). Several studies along these
lines have already been undertaken (e.g., Browder 1993, Manickchand-Heileman et al.
1998, Arreguín-Sánchez et al. 2004, Vidal-Hernandez & Pauly 2004).
Excessive bycatch and discards are associated with the shrimp trawl fishery, in which
small mesh nets are used. The 10:1 ratio of bycatch to shrimp implies that vast
quantities of non-target species are caught in shrimp trawls. Juveniles of sciaenids (e.g.,
croaker, seatrout, spot) constitute the bulk of the finfish bycatch, with many billion
individuals discarded every year (NOAA/NMFS 1999). The populations of species that
are heavily fished as bycatch in the shrimp fishery have declined significantly, in parallel
with the increase in shrimping effort (Brown et al. 1991). This loss through bycatch may
slow the recovery of overfished stocks (NOAA/NMFS 1999). Results from mass-balance,
trophic models suggest the ecosystem is rather robust as a whole, although continued
increases in fishing effort, especially by bottom (shrimp) trawlers, will have serious
impacts, reverberating through the entire shelf subsystem (Vidal-Hernandez & Pauly
2004).
III. Pollution and Ecosystem Health
Pollution: Shoreline development, the oil and gas industry, pollutant discharges and
nutrient loading are among the principal sources of stress on the Gulf of Mexico LME
(Birkett & Rapport 1999). In general, pollution was found to be slight to severe in this
LME. Most notable is the high input of nutrients and associated eutrophication and
hypoxia in the northern areas of the Gulf. Agricultural activities, artificial drainage and
680
50. Gulf of Mexico LME
other changes to the hydrology of the U.S. Midwest, atmospheric deposition, non-point
sources and point discharges, particularly from domestic wastewater treatment systems,
industrial discharges and feedlots all contribute to the nutrient load that reaches the Gulf
(Goolsby et al. 1999). The outflows of the Mississippi and Atchafalaya Rivers, however,
dominate the nutrient loads to the continental shelf (Rabalais et al. 1999a, 2002b). The
input of nutrients in the Mississippi River has increased dramatically in the last century
and has accelerated since 1950, coinciding with increasing fertiliser use in the Mississippi
Basin (Turner & Rabalais 1991). The high input and regeneration of nutrients result in
high biological productivity in the immediate and extended plume of the Mississippi River
(Lohrenz et al. 1990).
Most of the primary production fluxes to the bottom waters and the seabed, fuelling
hypoxia in the bottom waters. The areal extent of the hypoxic or `dead' zone during the
mid-summer of 1993-1995 ranged from about 16,600 to 18,200 km2 (Rabalais et al.
1999a, 1999b). The EPA predicted size of the dead zone by the end of summer 2007
was 22,127 km2 or more than 8,500 mi2. This is the largest zone of anthropogenic
coastal hypoxia in the western hemisphere. Evidence from chemical and biological
indicators in sediment cores shows the worsening hypoxic conditions in this LME
(Rabalais et al. 1996, 2002a). The U.S. EPA has classified the estuaries in the northern
Gulf as poor in terms of eutrophication (EPA 2001). In addition, HABs are of concern in
this LME. They debilitate fisheries for shellfish and affect tourism in Florida and Texas
(Anderson et al. 2000).
Inadequate management of sewage in the region has led to sewage contamination of
bays, lagoons and wetlands (Wong Chang & Barrera Escorcia 1996, Birkett & Rapport
1999). In some areas, microbiological pollution levels exceed permissible limits (Wong
Chang & Barrera Escorcia 1996). For example, high coliform levels (up to 300 faecal
coliforms MPN1/100
ml), greatly exceeding the sanitary regulation of 14
faecal
coliforms MPN/100 ml, have been detected in waters of Mecoacán, Tabasco and
Terminos Lagoons. In Galveston Bay, Texas, oysters have been severely affected by
pollution, and many public reefs have had to be closed due to organic pollution from
municipal sewage (Birkett & Rapport 1999).
Direct discharges and non-point sources of chemical pollutants are a major
environmental threat in the Gulf of Mexico LME (Birkett & Rapport 1999). The high use
of pesticides in agricultural areas has contributed to considerable levels of these
substances in the Mississippi and other rivers. These contaminants ultimately reach the
coastal waters. Heavy metals are released into the LME from numerous sources such as
municipal wastewater-treatment plants, manufacturing industries, mining and rural
agricultural areas. Elevated levels of heavy metals and pesticides have been detected in
water and sediments, in some cases exceeding permissible limits (Villaneuva Fragoso &
Paez-Osuna 1996, EPA 2001). The oil and gas industry has also had a significant
environmental and ecological impact on the LME (Botello et al. 1996, Birkett & Rapport
1999). Furthermore, the Gulf is a major thoroughfare for shipping, and accidental oil
discharges from tankers and oil installations are a constant threat. The Mississippi River
also delivers hydrocarbons to the Gulf, primarily from non-point source runoff. The
chronic exposure to oil residues from marine oil production is a significant source of
stress on the coastal habitats.
There is evidence of bioaccumulation of heavy metals, petroleum residues and PCBs in
the tissue of some finfish and invertebrate species (e.g., Botello et al. 1996, Villaneuva
Fragoso & Paez-Osuna 1996, Birkett & Rapport 1999, EPA 2001). In 2000, 10 out of
1 Most Probable Number
XV Wider Caribbean
681
14 fish consumption advisories for the coastal and marine waters of the northern Gulf
coast were issued for mercury, with each of the five US Gulf states having one state-wide
coastal advisory for mercury in king mackerel (EPA 2001). The widespread incidence of
fish diseases (e.g., lymphocytosis, ulcers, fin erosion, shell disease) thought to be related
to pollution has been reported in marine and estuarine species in the northern Gulf
(Birkett and Rapport 1999). The overall coastal condition for the U.S. part of this LME,
according to the EPA's primary indicators is: fair water quality, poor eutrophic condition,
poor condition of sediment and fish tissue (in terms of contaminants) and poor condition
of benthos (EPA 2001). In addition to the fish consumption advisories, the poor coastal
condition has also led to many beach closures throughout the northern Gulf coast, which
also has the lowest percentage of approved shellfish growing waters in the U.S. (EPA
2001).
Habitat and community modification: The LME's coastal and marine habitats are
threatened by both natural processes and anthropogenic factors and their modification is
severe throughout the LME (UNEP, unpublished). Hypoxia in the northern Gulf has
reduced the suitable habitat for living organisms and modified the benthic communities in
the affected area (Rabalais & Turner 2001). The more stressed community is
characterised by limited taxa, characteristic resistant fauna and severely reduced species
richness, abundances and biomass. The effects of hypoxia on fisheries resources
include direct mortality, altered migration, changes in food resources and disruption of life
cycles. Anectodal information from the 1950s to 1960s shows low or no catches by
shrimp trawlers from `dead' waters in this zone (Rabalais et al. 1999b).
Wetlands in particular have experienced severe loss and degradation due to coastal
development, interference with normal erosional/depositional processes, sea level rise
and coastal subsidence (EPA 2001). The EPA coastal wetlands indicator for the northern
Gulf of Mexico shows these wetlands to be in poor condition (EPA 2001). The periodic
sediment input to the Mississippi deltaic plain has been reduced by the construction of
flood control levees and dams upstream, the changing agricultural and urban water-use
practices and increasing alteration of the river system for navigation. The suspended
sediment load of the lower Mississippi decreased by about 50% during the period 1963-
1982 in response to dams built on the Arkansas and Missouri rivers (Meade 1995).
Wetlands are being converted to open water at an alarming rate because wetland
accretion is insufficient to compensate for the natural process of subsidence. In addition,
large areas of wetland have been drained for industrial, urban and agricultural
development. Wetland habitats are also being altered by increased salinities due to
saltwater intrusion, which is destroying coastal flora. This loss of wetlands also increases
erosion by waves and tidal currents and is exacerbated by sea level rise.
The effects of natural processes combined with human actions at large and small scales
have produced a system on the verge of collapse. Wetland losses in the U.S. Gulf of
Mexico from 1780s to 1980s are among the highest in the nation, with 50% having been
lost in this time period (EPA 2001). The rate of coastal land loss in Louisiana, which
contains the largest coastal wetland complex in the U.S., has reached catastrophic
proportions. Within the last 50 years, land loss rates have exceeded 104 km2yr-1,
representing 80% of the coastal wetland loss in the entire continental U.S. (Day et al.
2000).
In the coastal waters of the State of Campeche in Mexico, unregulated fishing, the use of
destructive fishing methods, as well as cutting of mangrove for aquaculture and other
purposes have destroyed fish habitats and reduced shrimp and other shellfish stocks
(Yañez Arancibia et al. 1999). The Usumacinta/Grijalva deltaic system is also being
modified because of changes in land use and the growing human population in this area.
682
50. Gulf of Mexico LME
As a consequence, coastal habitats and communities are being degraded and lost. For
example, the populations of some species such as the horseshoe crab (Limulus
polyphemus) and West Indian manatee (Trichechus manatus) have diminished as a
result of habitat and community modification in this system. Activities related to the oil
industry are also thought to have affected the distribution and abundance of commercially
important fisheries resources such as shrimp on the continental shelf and coastal
lagoons, particularly in the south of Tabasco and Campeche (Arreguín-Sánchez et al.
2004).
The LME's coral reefs are also threatened by natural and anthropogenic pressures.
Almost all the reefs of the Florida Keys are under moderate threat, largely from coastal
development, inappropriate agricultural practices, overfishing of target species such as
conch and lobster as well as pollution associated with development and farming (Bryant
et al. 1998). Other major threats in the last 20 years have arisen from direct human
impacts such as grounding of boats in coral, anchor damage and destructive fishing
(Causey et al. 2002). Reduced freshwater flow has resulted in increase of plankton
bloom, sponge and seagrass die-offs as well as the loss of critical nursery and juvenile
habitat for reef species, which affects populations on the offshore coral reefs. Serial
overfishing has dramatically altered fish and other animal populations. Alien species
introduced on the reefs in the last decade through ship hull fouling or ballast water
dumping have placed additional stress on the reefs (Causey et al. 2002).
Stresses from distant sources are also involved in the degradation of the region's reefs.
Waters from the Mississippi River periodically reach the Florida Keys while Saharan dust
has been implicated in the origin of nutrients and possibly disease spores, particularly
during El Niño years (Bryant et al. 1998). Florida reefs have been repeatedly stressed in
the past 25 years by coral bleaching, which has contributed to the dramatic declines in
coral cover in the Florida Keys National Marine Sanctuary since 1997 (Causey et al.
2002). Disease is also a serious problem. Two of the most important reef-building
species (Acropora palmata and A. cervicornis) are now relatively uncommon due to
white-band disease, while others have proved particularly susceptible to black-band
disease (Bryant et al. 1998). Algae continue to dominate all sites, with average cover
generally above 75% in the Keys and above 50% in the Dry Tortugas (Causey et al.
2002). The Flower Garden Banks off Texas, however, remain amongst the least
disturbed coral reefs in the region and can be considered nearly pristine. Nevertheless,
these reefs are threatened by atmospheric pollution and effluent discharges from nearby
oil and gas development and marine transportation. The reefs off Veracruz in the
southwestern gulf are influenced by high turbidity water from the coast and sewage and
other effluents from the port and city of Veracruz, resulting in low coral diversity. The
reefs on the Campeche Bank suffer from overfishing and the impacts of oil exploration
(Almada-Villela et al. 2002).
Seagrass habitats have declined dramatically during the past 50 years, mostly because
of coastal population growth and accompanying municipal, industrial and agricultural
development. In addition, boat propellers have permanently damaged over 120 km2 of
seagrass in the Florida Bay (Causey et al. 2002). Loss of seagrasses in the northern
Gulf of Mexico over the last five decades has been extensive and ranges from 20% to
100% for most estuaries, with only a few areas experiencing increases in seagrass.
Some experts believe that habitat loss is the greatest threat to the Gulf's biodiversity.
Unsustainable resource use is also contributing to species loss in this LME. In 2000, the
American Fisheries Society officially identified 11 of the Gulf's 15 managed grouper
species as `vulnerable to risk of extinction'. The only known nesting beach in the world of
the Kemp's ridley, the world's most endangered sea turtle, is along the Gulf of Mexico
coast. There has been considerable success, however, with the Ridley Head Start
XV Wider Caribbean
683
Program and establishment of nests along Padre Island in addition to Rancho Neuvo,
Tamaulipas, Mexico. Invasive species are also a major threat to biodiversity. Ballast
water discharges from transoceanic vessels are now known to be the single largest
source of introduction of aquatic non-indigenous species invasions worldwide and this
threat is particularly serious in the Gulf of Mexico LME, since the region contains some of
the world's largest ports (Nipper et al. 2005).
IV. Socioeconomic Conditions
The coastal areas of the LME are densely populated with about 55 million inhabitants.
This population is projected to increase by 144% between 1960 and 2010 (Cato & Adams
1999). The LME is a major economic asset to the three bordering countries, with the
value associated with various economic activities adding up to several billions of dollars
(Cato & Adams 1999, Adams et al. 2004). Commercial and recreational fisheries,
tourism and petroleum production are among the major economic activities. The Gulf is
also a major source of employment. For example, coastal employment in the five U.S.
Gulf states was more than 4 million in 1993 (Cato & Adams 1999).
In 2003, the U.S. domestic commercial landings from the Gulf amounted to about
800,000 tonnes valued at over US$680 million (NOAA/NMFS 2004). Nearly 3.3 million
instate marine recreational fishers made about 23 million trips and caught over
160 million fish (excluding Texas) in 2003 (Gulf of Mexico Program 2002). The Gulf
accounts for 30% of the U.S. offshore oil production and about 23% of its gas production.
More than 80% of the economic activities of each of the six Mexican Gulf states are
located in or associated with the coastal zone. These states contribute 12.9% of the total
national gross internal product (Sánchez-Gil et al. 2004). The tourist industry
encompasses thousands of businesses and tens of thousands of jobs worth well over
20 billion US$ annually (Gulf of Mexico Program 2002). Major port facilities and shipping
lanes exist in the LME.
Many important ecosystem services derived from the LME are threatened or have already
been lost (Birkett & Rapport 1999), with severe socioeconomic consequences.
Overexploitation of fisheries has resulted in deteriorating quantity and quality of the
catches and the imposition of restrictions and quotas (Birkett & Rapport 1999).
Overfishing has also led to reduced revenue from fisheries, user conflicts and loss of
employment in the affected states. The socioeconomic impacts of pollution as well as
habitat modification and loss are also severe. Analyses of the distribution of shrimp catch
on the shelf in relation to hypoxia suggest that the catch of shrimp was consistently low
where hypoxia was extensive (Zimmerman & Nance 2001). On the other hand, to date,
there are no clear indications of hypoxic effects in fisheries or fish populations in the
published literature or data evaluated by Diaz & Solow (1999). Nevertheless, the lack of
obvious detrimental economic effects does not preclude the possibility of future
ecological and economic disaster, as seen in other water bodies (e.g., the Black Sea)
affected by hypoxia (Diaz & Solow 1999).
Of particular concern are the potential health risks posed by marine biotoxins and HABs,
fish and shellfish poisoning and pollution. The contamination of seafood by pesticides
and heavy metals has led to loss in revenue from the closure of harvesting areas,
consumption advisories and risk to human health. This has been accompanied by an
increase in the costs of monitoring programmes and ecosystem protection and recovery.
Human society and its infrastructure in the coastal zone of the Gulf have already been
affected by wetland loss and will face considerably more threats as additional wetlands
are lost. Increased vulnerability to storm surge, coastal flooding and shoreline erosion
will result in damage to homes and loss of transportation and industrial infrastructure as
well as long-term degradation of critical resources such as domestic and industrial water
684
50. Gulf of Mexico LME
supplies. Coastal wetland deterioration will be devastating to culturally based
subsistence users as well as the recreational and tourist economies based on these
resources. If the recent loss of wetlands continues, it is estimated that Louisiana will lose
about 2,500 km2 more of coastal marshes, swamps and islands by 2050. The public use
value of this loss is estimated to be in excess of US$37 billion by year 2050; the losses
associated with cultures and heritage is immeasurable (Louisiana Coastal Wetlands
Conservation 1998). Major efforts at addressing the degradation of the Gulf of Mexico
LME (see Governance) are expected to reduce or reverse the current trends.
V. Governance
There is a multitude of programmes and policies to protect, restore and enhance the
coastal and marine waters and habitats of the Gulf of Mexico LME. For example, the
EPA's Gulf of Mexico Program, established in 1988, is conducting research, monitoring,
restoration and management projects in selected sites through its National Estuary
Program's Habitat Restoration Program and Gulf Ecological Management Sites Program.
In 2001, the EPA sent to Congress the final `Action Plan for Reducing, Mitigating and
Controlling Hypoxia in the Northern Gulf of Mexico'. This Action Plan was the culmination
of work undertaken by the Mississippi River/Gulf of Mexico Watershed Nutrient Task
Force and establishes the blueprint for addressing the hypoxia problem. The U.S. Gulf
Restoration Network and the Gulf of Mexico Foundation are engaged in various
programmes and projects aimed at protecting and restoring the Gulf's valuable resources
(e.g., CWPPRA, CIAP. The National Marine Fisheries Service (NMFS) Southeast
Regional Office is responsible for sustainable fisheries management, habitat
conservation and protected resources management (Kemmerer et al. 1999). This office
provides technical and administrative support to the Gulf of Mexico Fisheries
Management Council.
In Mexico, the Programme of Ecology, Fisheries and Oceanography of the Gulf of Mexico
(EPOMEX) was created in 1990 by the Autonomous University of Campeche. The main
focus of this programme is to generate and integrate information for the proposal of
management measures, development plans, ecological protection ranking, conservation
and sustainable use of coastal marine ecosystems and their natural resources in the gulf.
GEF is supporting the project `A Transboundary Diagnostic Analysis (TDA) and Strategic
Action (SAP) Programme for the Gulf of Mexico Large Marine Ecosystem' involving
Cuba, Mexico and the U.S. The main objective of this project is to address critical threats
to the coastal as well as marine environment and to promote ecosystem-based
management of coastal and marine resources in the Gulf of Mexico LME. The expected
outputs of this project will be a TDA and the development of a regional SAP for the LME.
The full GEF intervention will address the priority transboundary and biodiversity
concerns of the Gulf of Mexico LME in the context of fluctuating climate conditions.
References
Adams, C.M., Hernandez, E. and Cato, J.C. (2004). The economic significance of the Gulf of
Mexico related to population, income, employment, minerals, fisheries and shipping. Ocean
and Coastal Management 47(11-12):565-580.
Almada-Villela, P., Mcfield, M., Kramer, P., Richards Kramer, P. and Arias-González, E. (2002).
Status of coral Rrefs of Mesoamerica Mexico, Belize, Guatemala, Honduras, Nicaragua and
El Salvador, p 303-324 in: Wilkinson, C.R. (ed), Status of Coral Reefs of the World 2002.
Australian Institute of Marine Science, Townsville, Australia.
XV Wider Caribbean
685
Anderson, D.M., Hoagland, P., Kauru, Y. and White, A.W. (2000). Estimated Annual Economic
Impacts from Harmful Algal Blooms (HABs) in the US. Woods Hole Oceanographic Institute,
Woods Hole, U.S.
Arreguín-Sánchez, F., Sánchez, J.A., Flores Hernández, D., Ramos-Miranda, J., Sánchez-Gil, P.
and Yañez-Arancibia, A. (1999). Stock-recruitment relationships (SRRS): A scientific challenge
to support fisheries management in the Campeche Bank, Mexico, p 225 235 in: Kumpf, H.,
Steidinger, K. and Sherman, K. (eds), The Gulf of Mexico Large Marine Ecosystem
Assessment, Sustainability and Management. Blackwell Science, Malden, U.S.
Arreguín-Sánchez, F., Schultz-Ruiz, L.E., Gracia, A., Sánchez, J.A. and Alarcón, T. (1997). Estado
actual y perspectiva de las pesquerías de camarón, p 185-203 in: Flores-Hernández, D.,
Sánchez-Gil, P., Seijo, J.C. and Arreguín-Sánchez, F. (eds), Análisis y Diagnostico de los
Recursos Pesqueros Criticos del Golfo de Mexico. Ecology, Fisheries and Oceanography of the
Gulf of Mexico Serie Cientifica, Universidad Nacional Autonoma. Campeche, Mexico.
Arreguín-Sánchez, F., Zetina, M., Manickchand-Heileman, S. and Vidal, L. (2004). Simulated
responses to harvesting strategies in an exploited ecosystem in the southwestern Gulf of
Mexico. Ecological Modelling 172:421-432.
Baisre, J. (2000). Chronicle of Cuban marine fisheries (1935-1995):Trend analysis and fisheries
potential. FAO Fisheries Technical Paper 394, 39 p.
Belkin, I.M. (2008) Rapid warming of Large Marine Ecosystems, Progress in Oceanography, in
press.
Belkin, I.M., Cornillon, P.C., and Sherman, K. (2008). Fronts in Large Marine Ecosystems of the
world's oceans. Progress in Oceanography, in press.
Birkett, S.H. and Rapport, D.J. (1999). A stress-response assessment of the northwestern Gulf of
Mexico Ecosystem, p 438 458 in: Kumpf, H., Steidinger, K. and Sherman, K. (eds), The Gulf
of Mexico Large Marine Ecosystem Assessment, Sustainability and Management. Blackwell
Science, Malden, U.S.
Botello, A.V., Ponce Valez, G. and Macko, S.A. (1996). Niveles de concentracion de hidrocarburos
en el Golfo de Mexico, p 225-253 in: Botello, A.V., Rojas Galaviz, J.L., Benitez, J.A. and Zarate
Lomeli, D. (eds), Golfo de Mexico, Contaminacion e Impactos Ambiental: Diagnosticos y
Tendencias. Universidad Nacional Autonoma. Campeche, Mexico. Ecology, Fisheries and
Oceanography of the Gulf of Mexico Serie Cientifica 5.
Browder, J.A. (1993). A pilot model of the Gulf of Mexico continental shelf, p 279-284 in:
Christensen, V. and Pauly, D. (eds), Trophic Models of Aquatic Ecosystems. International
Centre for Living Aquatic Resources Management Conference Proceedings 26.
Brown, B.E., Browder, J., Powers, J. and Goodyear, C. (1991). Biomass, yield models and
management strategies for the Gulf of Mexico Ecosystem, p 534 564 in: Sherman, K.,
Alexander, L.M. and Gold, B.D. (eds), Large Marine Ecosystems: Food Chains, Yields, Models
and Management of Large Marine Ecosystems. Westview Press, Boulder, Colorado, U.S.
Bryant, D., Burke, L., McManus, J.W. and Spalding, M. (1998). Reefs at Risk: A Map-based
Indicator of Threats to the World's Coral Reefs. World Resource Institute, Washington D.C.,
U.S.
Cato, J. and Adams, C.M. (1999). Economic significance of the Gulf of Mexico related to
population, income, employment, minerals, fisheries and shipping, p 14 - 33 in: Kumpf, H.,
Steidinger, K. and Sherman, K. (eds), The Gulf of Mexico Large Marine Ecosystem
Assessment, Sustainability and Management. Blackwell Science, Malden U.S.
Causey, B., Delaney, J., Diaz, E., Dodge, D., Garcia, J., Higgins, J., Keller, B., Kelty, R., Jaap, W.,
Matos, C., Schmahl, G., Rogers, C., Miller, M. and Turgeon, D. (2002). Status of coral reefs in
the U.S. Caribbean and Gulf of Mexico: Florida, Texas, Puerto Rico, US Virgin Islands,
Navassa, p 251-276 in: Wilkinson, C.R. (ed), Status of Coral Reefs of the World 2002.
Australian Institute of Marine Science, Townsville, Australia.
Day, J. Shaffer, G.P., Britsch, L.D., Reed, D.J., Hawes, S.R. and Cahoon, D. R. (2000). Pattern
and process of land loss in the Mississippi Delta: A spatial and temporal analysis of wetland
habitat change. Estuaries, 23:425-438.
Díaz, R.J. and Solow, A. (1999). Ecological and Economic Consequences of Hypoxia: Topic 2.
Report for the Integrated Assessment on Hypoxia in the Gulf of Mexico. NOAA Coastal Ocean
Program Decision Analysis Series 16. National Oceanic and Atmospheric Administration,
Coastal Ocean Program, Silver Spring, U.S.
Emanuel, K. (2005), Increasing destructiveness of tropical cyclones over the past 30 years, Nature,
online publication; published online 31 July 2005 | doi: 10.1038/nature03906.
EPA (2001). National Coastal Condition Report. U.S Environmental Protection Agency, Washington
D.C., U.S.
686
50. Gulf of Mexico LME
www.epa.gov/gmpo/pdf/2007-hypoxia-pred-web-5.pdf for 2007 dead zone size prediction
FAO (2003). Trends in Oceanic Captures and Clustering of Large Marine Ecosystems: Two Studies
Based on the FAO Capture Database. FAO Fisheries Technical Paper 435.
Goolsby, D.A., Battaglin, W.A., Lawrence, G.B., Artz, R.S., Aulenbach, B.T., Hooper, R.P., Keeney,
D.R. and Stensland, G.J. (1999). Flux and Sources of Nutrients in the Mississippi-Atchafalaya
River Basin, Topic 3: Report for the Integrated Assessment of Hypoxia in the Gulf of Mexico.
NOAA Coastal Ocean Program Decision Analysis Series 17, National Oceanic and
Atmospheric Administration, Coastal Ocean Program, Silver Springs, U.S.
Gracia, A. and Vásquez-Bader, A.R. (1999). Shrimp fisheries in the south Gulf of Mexico: Present
and future management alternatives, p 205 224 in: Kumpf, H., Steidinger, K. and Sherman, K.
(eds), The Gulf of Mexico Large Marine Ecosystem Assessment, Sustainability and
Management. Blackwell Science, Malden, U.S.
Gulf of Mexico Program (2002). A Gulf of Mexico Program Fact Sheet. http://www.epa.gov/gmpo/
Huh, O. K., W. J. Wiseman, Jr. and Rouse, L.J., Jr. (1978). Winter cycle of sea surface thermal
patterns, northeastern Gulf of Mexico. Journal Geophysical Research 83(C9): 4523-4529.
Kemmerer, A., Cranmore, G. and Mager, A., Jr. (1999). Role of the National Marine Fisheries
Service in natural resource management in the Gulf of Mexico Large Marine Ecosystem, p 678
691 in: Kumpf, H., Steidinger, K. and Sherman, K. (eds), The Gulf of Mexico Large Marine
Ecosystem Assessment, Sustainability and Management. Blackwell Science, Malden, U.S.
Kumpf, H., Steidinger, K. and Sherman, K., eds. (1999). The Gulf of Mexico Large Marine
Ecosystem Assessment, Sustainability and Management. Blackwell Science, Malden, U.S.
Lohrenz, S.E., Dagg, M.J. and Whitledge, T.E. (1990). Enhanced primary production at the
plume/oceanic interface of the Mississippi River. Continental Shelf Resources. 10:639-664.
Lohrenz, S.E., Wiesenburg, D.A., Arnone, R.A. and Chen, X. (1999). What controls primary
production in the Gulf of Mexico? p 151 170 in: Kumpf, H., Steidinger, K. and Sherman, K.
(eds), The Gulf of Mexico Large Marine Ecosystem Assessment, Sustainability and
Management. Blackwell Science, Malden, U.S.
Louisiana Coastal Wetlands Conservation (1998). Louisiana Coastal Wetlands Conservation and
Restoration Task Force and the Wetlands Conservation and Restoration Authority. Coast 2050:
Toward a Sustainable Coastal Louisiana, an Executive Summary. Louisiana Department of
Natural Resources, Baton Rouge, Lousiana, U.S.
Manickchand-Heileman, S., Soto, L.A. and Escobar, E. (1998). A preliminary trophic model of the
continental shelf, southwestern Gulf of Mexico. Estuarine, Coastal and Shelf Science 46(6):885-
899.
Meade, R.H., ed. (1995). Contaminants in the Mississippi River, 1987-1992. U.S. Geological
Survey Circular 1133, US Department of the Interior US Geological Survey, Denver, U.S.
Nipper, M., Sánchez Chávez, J.A. and Tunnell, J.W., eds. (2005). GulfBase: Resource Database
for Gulf of Mexico Research. www.gulfbase.org
NOAA/NMFS (1999). Our Living Oceans: Report on the Status of US Living Marine Resources.
National Marine Fisheries Service / National Oceanic and Atmospheric Administration.
NOAA/NMFS (2004). Fisheries of the U.S 2003. http://www.st.nmfs.gov/st1/fus/fus03/index.html
Pauly, D. and Christensen, V. (1995). Primary production required to sustain global fisheries.
Nature 374: 255-257.
Pauly, D. and Watson, R. (2005). Background and interpretation of the `Marine Trophic Index' as a
measure of biodiversity. Philosophical Transactions of the Royal Society: Biological Sciences
360: 415-423.
Pauly, D., Arreguín-Sánchez, F., Browder, J., Christensen, V., Manickchand-Heileman, S.,
Martínez, E. and Vidal, L. (1999). Toward a stratified mass-balance model of trophic fluxes in
the Gulf of Mexico Large Marine Ecosystem, p 278-293 in: Kumpf, H., Steidinger, K. and
Sherman, K. (eds), The Gulf of Mexico Large Marine Ecosystem Assessment, Sustainability
and Management. Blackwell Science, Malden, U.S.
Pauly, D., Christensen, V., Dalsgaard, J., Froese R. and Torres, F.C. Jr. (1998). Fishing Down
Marine Food Webs. Science 279: 860-863.
Rabalais, N.N. and Turner, R.E., eds. (2001). Coastal hypoxia: Consequences for living resources
and ecosystems. Coastal and Estuarine Studies 58, American Geophysical Union, Washington
D.C., U.S.
Rabalais, N.N., Turner, R.E. and Scavia, D. (2002a). Beyond science into policy: Gulf of Mexico
hypoxia and the Mississippi River. BioScience 52:129-142.
Rabalais, N.N., Turner, R.E. and Wiseman, W.J. (1999a). Hypoxia in the northern Gulf of Mexico:
Linkages with the Mississippi River, p 297-322 in: Kumpf, H., Steidinger, K. and Sherman, K.
XV Wider Caribbean
687
(eds), The Gulf of Mexico Large Marine Ecosystem Assessment, Sustainability and
Management. Blackwell Science, Malden, U.S.
Rabalais, N.N., Turner, R.E., Dortch, Q., Justi, D., Bierman, V.J. and Wiseman, W.J. (2002b).
Review. Nutrient-enhanced productivity in the northern Gulf of Mexico: Past, present and future.
Hydrobiologia 475/476:39-63.
Rabalais, N.N., Turner, R.E., Justic, D., Dortch, Q. and Wiseman, W.J. (1999b). Characterisation of
Hypoxia: Topic 1. Report for the Integrated Assessment on Hypoxia in the Gulf of Mexico.
NOAA Coastal Ocean Program Decision Analysis Series 15. National Oceanic and
Atmospheric Administration, Coastal Ocean Program, Silver Spring, U.S.
Rabalais, N.N., Turner, R.E., Justi, D., Dortch, Q., Wiseman W.J. and Sen Gupta, B.K. (1996).
Nutrient changes in the Mississippi River and system responses on the adjacent continental
shelf. Estuaries 19(2B):386-407.
Richards, W. and McGowan, M.F. (1989). Biological productivity in the Gulf of Mexico: Identifying
the causes of variability in fisheries, p 287 325 in: Sherman, K. and Alexander, L.M. (eds),
Biomass Yields and Geography of Large Marine Ecosystems, Westview Press, Boulder, U.S.
Sánchez-Gil, P., Yáñez-Arancibia, A., Ramírez-Gordillo, J., Day, J.W. and Templet, P.H. (2004).
Some socio-economic indicators in the Mexican states of the Gulf of Mexico. Ocean and
Coastal Mangement 47 (11-12):581-596.
Sea Around Us (2007). A Global Database on Marine Fisheries and Ecosystems. Fisheries Centre,
University British Columbia, Vancouver, Canada. www.seaaroundus.org/lme/
SummaryInfo.aspx?LME=5
Sherman, K. (2003). Physical, Biological and Human Forcing of Biomass Yields in Large Marine
Ecosystems. ICES CM 2003/P: 12.
Shipp, R. (1999). Status of exploited fish species in the Gulf of Mexico, p 196 204 in: Kumpf, H.,
Steidinger, K. and Sherman, K. (eds), The Gulf of Mexico Large Marine Ecosystem
Assessment, Sustainability and Management. Blackwell Science, Malden, U.S.
Turner, R.E. and Rabalais, N.N. (1991). Changes in the Mississippi River water quality this
Century. Implications for coastal food webs. BioScience 41(3):140-147.
UNDP/GEF (2004). Project Document. A Transboundary Diagnostic Analysis and Strategic Action
Program for the Gulf of Mexico Large Marine Ecosystem.
Vidal-Hernandez, L. and Pauly, D. (2004). Integration of subsystem models as a tool toward
describing feeding interactions and fisheries impacts in a Large Marine Ecosystem, the Gulf of
Mexico. Ocean and Coastal Management 47: 709-725.
Villaneuva Fragoso, S. and Páez-Osuna, F. (1996). Niveles de metales en el Golfo de Mexico:
Agua, sedimentos y organismos, p 309-347 in: Botello, A.V., Rojas Galaviz, J.L., Benitez, J.A.,
Zarate Lomeli, D. (eds), Golfo de Mexico, Contaminacion e Impactos Ambiental: Diagnosticos y
Tendencias. Univ. Auton. Campeche, Ecology, Fisheries and Oceanography of the Gulf of
Mexico Serie Cientifica 5.
Wiseman, W.J. and Sturges, W. (1999). Physical oceanography of the Gulf of Mexico: Processes
that regulate its biology, p 77 92 in: Kumpf, H., Steidinger, K. and Sherman, K. (eds), The Gulf
of Mexico Large Marine Ecosystem Assessment, Sustainability and Management. Blackwell
Science, Malden, U.S.
Wong Chang, I. and Barrera Escorcia, G. (1996). Niveles de contaminacion microbiologica en el
Golfo de Mexico, p 383-397 in: Botello, A.V., Rojas Galaviz, J.L., Benítez, J.A., Zarate Lomeli,
D. (eds), Golfo de Mexico, Contaminacion e Impactos Ambiental: Diagnosticos y Tendencias.
Universidad Nacional Autonoma. Campeche, Mexico. Ecology, Fisheries and Oceanography of
the Gulf of Mexico Serie Cientifica 5.
Yañez Arancibia, A., Lara-Domínguez, A.L., Rojas Galaviz, J.L., Villalobos Zapata, G.L., Zarate,
D.J. and Sánchez-Gil, P. (1999). Integrated coastal zone management plan for Terminos
Lagoon, Campeche, Mexico, p 565 592 in: Kumpf, H., Steidinger, K. and Sherman, K. (eds),
The Gulf of Mexico Large Marine Ecosystem Assessment, Sustainability and Management.
Blackwell Science, Malden, U.S.
Yañez-Arancibia, A. (ed.) (1985). Recursos Pesqueros Potenciales de Mexico: la Pesca
Acompañante del Camaron. Progr. Univ. de Alimentos/Inst. Cienc. del Mar y Limnol./Inst. Nal.
de Pesca/UNAM, Mexico D.F., 768 p.
Zimmerman, R.J. and Nance, J.M. (2001). Effects of hypoxia on the shrimp fishery of Louisiana
and Texas, p 293-310 in: Rabalais, N. N. and Turner, R. E. (eds), Coastal Hypoxia:
Consequences for Living Resources and Ecosystems. Coastal and Estuarine Studies 58,
American Geophysical Union, Washington D.C., U.S.
688
50. Gulf of Mexico LME
XV Wider Caribbean
689
XV-51 Southeast U.S. Continental Shelf LME
M.C. Aquarone
The Southeast U.S. Continental Shelf LME extends from the Straits of Florida to Cape
Hatteras, North Carolina in the Atlantic Ocean. It is characterised by its temperate
climate. The LME has a surface area of about 300,000 km², of which 2.44% is protected,
and contains 0.27% of the world's coral reefs and 18 estuaries and river systems (Sea
Around Us 2007). It also contains many bays including the Albemarle-Pamlico Sound,
the second largest estuary in the nation, nearshore and barrier islands, freshwater and
estuarine habitats and extensive coastal marshes that provide unique habitats for living
marine resources. A book chapter pertaining explicitly to this LME is by Yoder (1991).
I. Productivity
The Southeast U.S. Continental Shelf LME is considered a Class I, highly productive
ecosystem (>300 gCm-2yr-1). Additional information is provided by NOAA statistics in Our
Living Oceans (NOAA 1999). A chapter on marine resources for the southeast region
(with the Gulf of Mexico LME and Caribbean islands), including information on status and
trends of the nation's biological resources, primary and secondary productivity, benthic
resources, fisheries resources, marine birds and marine mammals can be found at the
USGS biology website. The North Carolina Albemarle-Pamlico Sound is one of the
largest and most productive aquatic systems in North America. Upwelling along the Gulf
Stream front and intrusions from the Gulf Stream cause short-lived plankton blooms. The
offshore upwelling regime is not as intense as in the higher latitude regions (see Yoder
1991).
Oceanic Fronts: Adjacent to this LME, the warm, saline, northward flowing Gulf Stream
is bounded by two fronts (Figure XV-51.1). The inshore Gulf Stream Front (IGSF)
extends over the upper continental slope and shelf break, approximately aligned with the
50-m isobath (Atkinson & Menzel 1985), while the offshore Gulf Stream Front (OGSF)
runs parallel to the IGSF, approximately 100 km offshore of the latter.
This LME is radically different from the Northeast U.S. Continental Shelf LME, where the
Shelf-Slope/Shelf Break Front is associated with a cold, fresh southward Slope Current.
The Gulf Stream forms a semi-permanent offshore deflection near a deepwater bank SE
of Charleston, NC, called the `Charleston Bump' (CB), at 31.5°N in the Southeast Shelf
LME. The Mid-Shelf Front (MSF) is aligned approximately with the 35-to-40 meter
isobaths. Other shelf fronts separate a mixture of water masses formed by wintertime
cold air outbreaks, river discharge, tidal mixing and wind-induced coastal upwelling
(Pietrafesa et al. 1985, Belkin et al. 2005).
U.S. Southeast Shelf LME SST
Linear SST trend since 1957: -0.15°C.
Linear SST trend since 1982: 0.16°C.
The Southeast US Continental Shelf is one of a few LMEs that experienced long-term
cooling since 1957. Like most LMEs, the Southeast US Continental Shelf first went
through a cooling phase before switching to a warming phase in 1976. Warming over the
last 25 years was small, just 0.16°C. Given 1976 as a well-defined break point, this
warming would amount to 0.5°C. The 1976 breakpoint could be tentatively associated
with a similar break point in 1976 in the Gulf of Mexico LME, however the latter breaking
point is not well defined. Nonetheless, the possible
























































































690
U.S. Southeast Continental Shelf LME
Figure XV-51.1. Fronts of the Southeast U.S. Continental Shelf LME. CB, Charleston Bump; IGSF,
Inshore Gulf Stream Front; MSF, Mid-Shelf Front; OGSF, Offshore Gulf Stream Front. Yellow line, LME
boundary. After Belkin et al. 2008.
link between these LMEs cannot be dismissed since they are connected by the Gulf
Stream flowing from the Gulf of Mexico past the Southeast US Shelf. Therefore,
advection of SST anomalies from the Gulf of Mexico to the Southeast US Shelf is
expected to play a key role in the thermal regime of the Southeast US Shelf. The two
major SST peaks of 1961 and 1975 did not have immediate upstream precursors in the
Gulf of Mexico. The 3-year time lag between the Gulf of Mexico SST peak of 1972 and
the Southeast US Shelf SST peak of 1975 makes this connection tenuous. On the other
hand, the 3-year time lag between the Gulf of Mexico and the Southeast US Shelf is
consistent with the 3-year time lag between the Caribbean LME and the Gulf of Mexico.
Figure XV-51.2. Southeast US Shelf LME annual mean SST and annual SST anomalies, 1957-2006,
based on Hadley climatology. After Belkin 2008.
XV Wider Caribbean
691
U.S. Southeast Shelf LME Chlorophyll and Primary Productivity: The Southeast
U.S. Continental Shelf LME is considered a Class I, highly productive ecosystem (>300
gCm-2yr-1).
Figure XV-51.3. U.S. Southeast shelf LME trends in chlorophyll a and primary productivity, 1998-2006.
Values are colour coded to the right hand ordinate. Figure courtesy of J. O'Reilly and K. Hyde. Sources
discussed p. 15 this volume.
II. Fish and Fisheries
The estuaries support diverse aquatic organisms and complex food webs in a nursery
system that promotes the recruitment and development of juvenile fish and invertebrate
species important to recreational, commercial, and ecological interests (EPA 2004). The
major species are coastal pelagics (mackerel, dolphinfish, and cobia), highly migratory
pelagics (swordfish, tuna, albacore, marlin, sailfish, spearfish and sharks), Atlantic
menhaden, invertebrates (shrimp, lobster, crab and conch), reef fish, drum and croaker,
and Atlantic sharks. Major species landed include the coastal pelagic species, highly
valued and sought after as game fish, the Atlantic highly migratory pelagic fish (especially
yellowfin tuna), menhaden, and white and northern brown shrimps, centered off Georgia
and the Carolinas. Shrimp stocks are affected by environmental conditions and by
increased fishing pressure (NMFS 2009). Total reported landings increased from 1950,
recording over 150,000 tonnes in 1981 and 1984, but have since declined to 62,000
tonnes in 2004 (Figure XV-51.4). There are major fluctuations in the landings of Atlantic
menhaden, with peaks in the 1950s, drops in the late 1960s, another peak in 1983,
followed by less than 2,000 tonnes landed in 1984 and 1997. Combined commercial and
recreational landings of reef fishes have fluctuated since the 1970s, showing a slightly
decreasing trend over time (EPA 2004). The value of the reported landings for the
Southeast US Continental Shelf LME reached almost 400 million US$ (measured in year
2000 US$) in 1979, two-thirds of which was from the landings of crustaceans (Figure XV-
51.5).
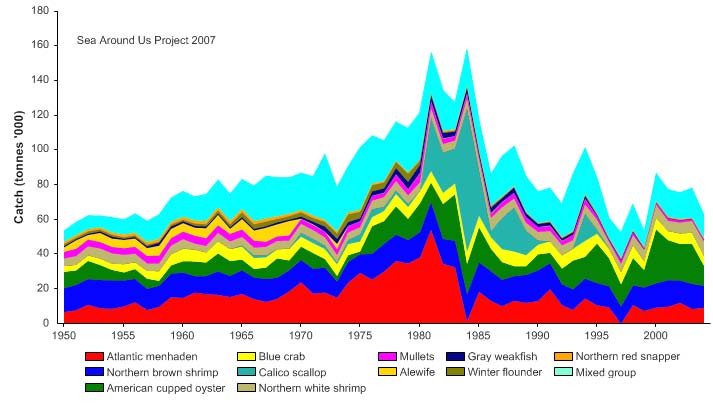
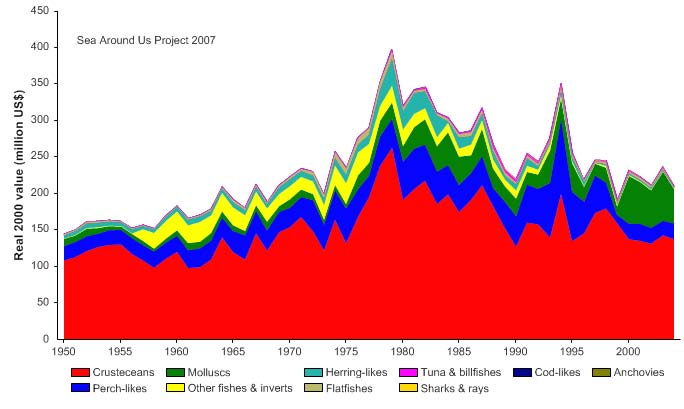
692
U.S. Southeast Continental Shelf LME
Figure XV-51.4. Total reported landings in the Southeast U.S. Continental Shelf LME by species (Sea
Around Us 2007).
Figure XV-51.5. Value of reported landings in the Southeast U.S. Continental Shelf LME by commercial
groups (Sea Around Us 2007).
The primary production required (PPR) (Pauly & Christensen 1995) to sustain the
reported landings in the LME reached 6.5% of the observed primary production in 1980
but has not reached this level since (Figure XV-51.6. The US accounts for the largest
share in this LME of the ecological footprint measured as the primary production required
to support reported landings by countries.
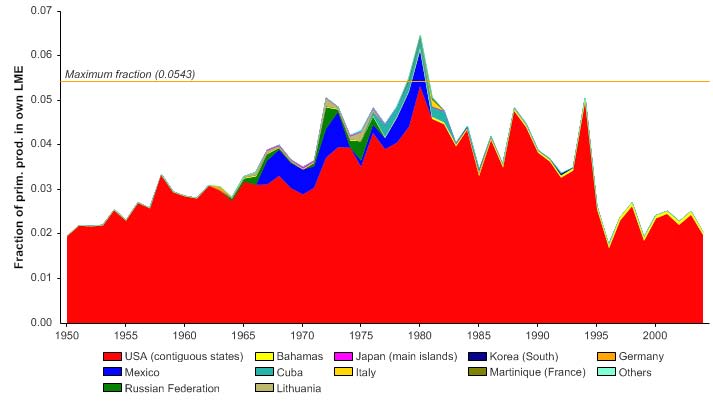
XV Wider Caribbean
693
Figure XV-51.6. Primary production required to support reported landings (i.e., ecological footprint) as
fraction of the observed primary production in the Southeast U.S. Continental Shelf LME (Sea Around
Us 2007). The `Maximum fraction' denotes the mean of the 5 highest values.
The mean trophic index (MTI) of the reported landings (Pauly & Watson 2005) shows a
decreasing mean trophic level, though with some fluctuations (Figure XV-51.7 top). The
trend becomes more pronounced when tuna landings are excluded and examined at a
local level (see Figure 4 in Chuenpagdee et al. 2006). With the FiB index also declining
sharply since the mid 1970s (Figure XV-51.7 bottom), the state of the LME can be
diagnosed as undergoing a `fishing down' of the food web (Pauly et al. 1998) with no
increase in the landings to compensate for the decline in the mean trophic level of the
catch.
Figure XV-51.7. Mean trophic level (i.e., Marine Trophic Index) (top) and Fishing-in-Balance Index
(bottom) in the Southeast U.S. Continental Shelf LME (Sea Around Us 2007).
694
U.S. Southeast Continental Shelf LME
The Stock-Catch Status Plots indicate that collapsed and overexploited stocks now
account for over 80% of all commercially exploited stocks in the LME (Figure XV-51.8,
top), with fully exploited stocks contributing more than half of the catch (Figure XV-51.8,
bottom). The US National Marine Fisheries Service (NMFS) includes "overfished" but not
"collapsed" in its stock status categories. Currently overfished are reef fishes (grouper,
black sea bass, red porgy), highly migratory pelagic fisheries (albacore, blue marlin,
bluefin tuna, yellowfin tuna, and sailfish,) and sciaenids such as red drum in some states.
Bigeye tuna and swordfish are rebuilding (NMFS 2009). The populations of several
species of sciaenids, most notably Atlantic croaker, appear to be closely linked to
environmental conditions resulting in large annual fluctuations in population levels (EPA
2004). Removals of apex predators from the reef complex may result in shifts of species
composition (i.e. trophic and ecological cascades) and increased variability in population
dynamics of targeted species. Stock rebuilding plans are in effect for all reef fish species
classified as overfished. The latest NMFS catch statistics indicate that commercial
shrimp species are being harvested at maximum levels. Atlantic Spanish mackerel are
considered to be at or near their full maximum fishery potential. Following declines in the
abundance of large coastal sharks, new management measures were introduced in
1997.
0%
100
10%
90
20%
)
80
%
(
s
30%
u
70
at
st
40%
y
60
50%
cks b
50
o
f
st
60%
40
r
o
e
70%
mb
30
Nu
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 3233)
developing
fully exploited
over-exploited
collapsed
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
80
)
%
30%
(
70
s
u
at
40%
60
ck st
50%
o
50
st
y
60%
b
h
40
t
c
a
70%
C
30
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 3233)
developing
fully exploited
over-exploited
collapsed
Figure XV-51.8. Stock-Catch Status Plots for the Southeast U.S. Continental Shelf LME, showing the
proportion of developing (green), fully exploited (yellow), overexploited (orange) and collapsed (purple)
fisheries by number of stocks (top) and by catch biomass (bottom) from 1950 to 2004. Note that (n), the
number of `stocks', i.e., individual landings time series, only include taxonomic entities at species,
genus or family level. Higher and pooled groups have been excluded (see Pauly et al, this vol. for
definitions).
Our Living Oceans (NOAA 1999) has statistics on landings of blue crab, sea urchin and
oyster from the Atlantic coast, and landings and spawning biomass for menhaden from
1950 to 1997 (NOAA 1999, p. 141). The 2008 (quarterly) NOAA Status of U.S. Fisheries
XV Wider Caribbean
695
Report to Congress (www.noaa.gov) contains the status (fished or overfished) of selected
species. The annual report on fishery landings in the US provided by the NOAA-
Fisheries Office of Science and Technology can be found at www.st.nmfs.noaa.gov.
Information on large marine ecosystem fisheries is available in EPA 2004. This includes
reef fish resources (see graph of coast reef fish landings, 1978-2000) as well as sciaenid,
menhaden, mackerel and shrimp fisheries. The Georgia Department of Natural
Resources Red Drum Project highlights the importance of habitat for all life stages of red
drum (EPA 2004), an important fishery resource along the Atlantic coast since the late
1800s. Currently, these fish species support substantial harvests for both commercial and
recreational fisheries and are captured in almost every type of gear used to fish the
coastal waters of this LME.
III. Pollution and Ecosystem Health
The dynamic fringe of estuaries varies constantly with tidal fluctuations and levels of
runoff, and it serves as an important habitat for waterfowl, reptiles, mammals, fish and
invertebrates, as well as a diversity of plants. It also serves as a natural filter to remove
pollutants and sediments from upland regions (EPA 2004). Species such as shrimps,
crabs and menhaden, which account for much of the catch in this LME, are estuarine-
dependant. There are habitat concerns impacting many of the Southeast invertebrate
fishery resources. Additional studies are needed to further assess the impacts of human-
induced changes in habitat availability, environmental conditions, predator abundance,
and pollution in nursery areas. Florida spiny lobsters depend on reef habitat and shallow
water algal flats for feeding and reproduction, but these habitat requirements can conflict
with expanding coastal development in the region. The small mesh used in shrimp trawls
can catch non-target species such as sea turtles, red snappers, croakers, seatrouts, and
other species (NMFS 2009). All sea turtle species are listed as endangered or threatened
under the Endangered Species Act. Shrimp vessels are required to use turtle excluder
devices in their nets since 1988.
Of the four U.S. Continental Shelf LMEs, the Southeast U.S. Continental Shelf LME has
the best ecological condition. The U.S. EPA provides data on environmental stressors
(water quality, sediment quality and tissue bioaccumulation) throughout the U.S. See
EPA (2001, 2004) for the coastal condition of the Southeast region, which includes this
LME. In 2001, the index for dissolved oxygen and fish tissue condition was good. Water
clarity, coastal wetlands, eutrophic condition, sediment and benthos were fair (see EPA's
7 primary indicators in EPA 2001). The condition of the southeastern estuaries was fair.
Approximately 54% of estuarine areas are in good ecological condition (EPA 2001, EPA
2004), based on five primary indicators: water quality (rated fair to good); sediment
quality (rated fair to good); benthic index (fair); coastal habitat index (fair); and fish tissue
index (good). The Albemarle-Pamlico Estuarine System's resources are threatened by
increased pollution from urban and agricultural development in its watersheds (EPA
2004). For figures on coastal wetland habitat loss from 1780 to 1980, see EPA (2001).
By 1980, 40% of all wetlands existing in 1780 had disappeared.
The increasing population growth could contribute to increased water quality degradation
in this region. A primary problem is sediment contamination by pesticides and metals.
Municipal wastewater treatment plants and pesticides applied to agricultural lands are
sources of coastal pollution. NOAA's National Status and Trends program provides data
on toxic contaminants and their ecological effects. See EPA 2004 (www.epa.gov/) for
information on South Carolina's Estuarine and Coastal Assessment Program which
monitors the biological condition of 60 sites throughout the state's coastal zone (p.119),
comparing and predicting PAH concentrations in urban and non-urban settings in South
Carolina (p. 120), Clean Water Act assessments, and fish consumption and beach
advisories. In 2002, 15% of beached were affected by advisories or closures. The
696
U.S. Southeast Continental Shelf LME
reasons were pre-emptive closure because of rainfall (24%), or elevated bacteria levels
(75%).
IV. Socioeconomic Conditions
The Southeast U.S. Continental Shelf LME contains a wealth of resources including both
commercial and recreational fisheries. Bycatch of Atlantic highly migratory species, and
increasing numbers of recreational spiny lobster participants, cause conflicts between
commercial and recreational fisheries and reduce the impact of conservation efforts.
Other resources and economic activities in the LME include barrier islands such as North
Carolina's outer banks, and busy shipping ports in Miami and Jacksonville, Florida,
Savanna, Georgia, and Charleston, South Carolina. Non-consumptive uses of reef
resources (e.g. ecotourism, sport diving, education, and scientific research) are
economically important and may conflict with traditional commercial and recreational
fisheries. Balancing the competing interests of these user groups is an important
management issue.The Albemarle-Pamlico Sound is North Carolina's key resource base
for commercial and recreational fishing and tourism. This resource and other coastal
resources of the Southeast Coast states generate vast amounts of sales tax income for
those states (EPA 2004).
Fishing pressure has increased over time in correlation with growing human populations,
greater demands for sea food, and technological improvements in gear, electronic fish
finders, and navigational aids. The coastal population has shown a growth rate of almost
2% per year (EPA 2004). The population increase amounted to 64% between 1970 and
1990 (U.S. Census Bureau 1996). In 1999, the southern region of the U.S. was the most
populous area of the nation, accounting for 96 million residents. Florida was among the
five most populous states in 1999 (U.S. Census Bureau 2001). The influx of people and
businesses to this region, and added pressure on the coastal zone, will require additional
programs and more environmental awareness in order to correct existing problems of
ecosystem health.
V. Governance
The South Atlantic Fisheries Management Council (SAFMC) manages this LME's fish
stocks in collaboration with the NMFS Southeast Fisheries Centre within the US
Exclusive Economic Zone (EEZ), seaward of territorial waters out to 200 miles from the
shore. Coastal pelagic fishes are jointly managed under the Coastal Migratory Pelagic
Resources Fishery Management Plan and the regulations adopted by the SAFMC.
Management regulations have included total allowable catches (TACs) and minimum size
restrictions. Effective management of migratory coastal pelagic species will continue to
require the coordination of Federal and state regulatory agencies in North Carolina,
South Carolina, Georgia and Florida. US fleets for highly migratory pelagic fisheries
operating in this LME are regulated under the Magnuson-Stevens Fishery Conservation
and Management Act and the Atlantic Tunas Convention Act (ATCA). Management of
Atlantic tunas and swordfish in US waters are based largely on recommendations by the
International Commission for the Conservation of Atlantic Tunas (ICCAT). Some shark
species are included in the International Union for the Conservation of Nature (IUCN)
Red List of Threatened Species as vulnerable. Because Atlantic menhaden migrates long
distances, interstate coordination of fishery management is required (NMFS 2009).
Specific fishery management plans, including for the shrimp fishery, are available in Our
Living Oceans (NOAA 1999). MPAs are used as management tools for deepwater
species of reef fish. There is an increasing need for effective management of these
resources given the predicted influx of people to the LME boundary coastal states (EPA
2001).
XV Wider Caribbean
697
References
Atkinson, L. P. and Menzel, D.W. (1985) Introduction: Oceanography of the Southeastern U.S
Continental Shelf, p 1-9 in: Atkinson, L.P., Menzel, D.W. and Bush, K.A. (eds), Oceanography
of the Southeastern U.S. Continental Shelf, Coastal and Estuarine Sciences 2, American
Geophysical Union, Washington D.C., U.S.
Belkin, I.M. (2005) Oceanic Fronts in Large Marine Ecosystems, Final Report to the UNEP, 49 pp.,
64 maps.
Belkin, I.M., Cornillon, P.C., and Sherman, K. (2008). Fronts in large marine ecosystems of the
world's oceans: An atlas. Progress in Oceanography, submitted.
Chuengpagdee, R., Preikshot, D., Liguori, L. and Pauly, D. (2006). A public sentiment index for
ecosystem management. Ecosystems 9: 463-473.
EPA (2001). National Coastal Condition Report. Chapter 4.
EPA (2004). National Coastal Condition Report 2. EPA-620/R-03-002. Office of Research and
Development/Office of Water. Washington DC. 286 pages. Also available at:
www.epa.gov/owow/oceans/nccr2/
Hyland, J.L., Herrlinger, T.J., Snoots, T.R., Ringwood, A.H., Van Dolah, R.F., Hackney, C.T.,
Nelson, G.A., Rosen, J.S. and Kokkinakis, S.A. (1996). Environmental quality of estuaries of the
Carolinian Province: 1994.
NMFS. 2009. Our living oceans. Draft report on the status of U.S. living marine resources, 6th
edition. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-F/SPO-80. 353 p.
NOAA (1999). Our living oceans Report on the status of U.S. living marine resources. U.S.
Department of Commerce, NOAA Technical Memorandum. NMFS-F/SPO-41, 301 p. On the
web at: http://spo.nwr.noaa.gov/olo99.htm.
NOAA-Fisheries. The annual report on fishery landings in the US. Office of Science and
Technology. www.st.nmfs.noaa.gov.
NOAA Fisheries Report to Congress (2008) available quarterly at www.noaa.gov, (search
reports to Congress)
Pauly, D. and Christensen, V. (1995). Primary production required to sustain global fisheries.
Nature 374: 255-257.
Pauly, D. and Watson, R. (2005). Background and interpretation of the `Marine Trophic Index' as a
measure of biodiversity. Philosophical Transactions of the Royal Society: Biological Sciences
360: 415-423.
Pauly, D., Christensen, V., Dalsgaard, J., Froese R. and Torres, F.C. Jr. (1998). Fishing Down
Marine Food Webs. Science 279: 860-863.
Pietrafesa, L. J., Janowitz, G.S. and Wittman, P.A. (1985) Physical oceanographic processes in the
Carolina Capes, p 23 -32 in: Atkinson, L.P., Menzel, D.W. and Bush, K.A. (eds), Oceanography
of the Southeastern U.S. Continental Shelf, Coastal and Estuarine Sciences 2, American
Geophysical Union, Washington D.C.
Sea Around Us (2007). A Global Database on Marine Fisheries and Ecosystems. Fisheries Centre,
University British Columbia, Vancouver, Canada. www.seaaroundus.org
/lme/SummaryInfo.aspx?LME=6
Sherman, K. and Hoagland, P. (2005). Driving forces affecting resource sustainability in Large
Marine Ecosystems. ICES CM 2005/M:07
U.S. Census Bureau (1996). Population in Coastal Counties: April 1, 1990 and July 1, 1994.
Bureau of the Census, Population Distribution Branch, Washington, D.C. www.census.gov/
population /estimates/county/9094cstl.txt.
U.S. Census Bureau (2001). Current Population Reports, Series P23-205, Population Profile of the
U.S: America at the Close of the 20th Century. U.S. Government Printing Office, Washington,
D.C.
USGS biology at http://biology.usgs.gov/s+t/SNT/noframe/mr181.htm#86315
Yoder, J. A. (1991). Warm-temperate food chains of the southeast shelf ecosystem, p 49-66 in
Sherman, K., Alexander, L.M. and Gold, B.D. (eds), Food Chains, Yields, Models and
Management of Large Marine Ecosystems. AAAS Symposium. Westview Press, Inc., Boulder,
U.S.
698
U.S. Southeast Continental Shelf LME










































































































