VII South Asian Seas
236
10. Bay of Bengal LME
VII South Asian Seas
237
VII-10 Bay of Bengal LME
S. Heileman, G. Bianchi and S. Funge-Smith
The Bay of Bengal LME is a relatively shallow embayment in the northeastern Indian
Ocean encompassing the Bay of Bengal, Andaman Sea and Straits of Malacca. It is
bordered by Bangladesh, India, Indonesia, Malaysia, Maldives, Myanmar, Sri Lanka and
Thailand. The LME covers an area of about 3,660,130 km2, of which 0.49% is protected,
and contains 3.63% and 0.12% of the world's coral reefs and sea mounts, respectively
(Sea Around Us 2007). It is influenced by the second largest hydrologic region in the
world, the Ganges-Brahmaputra-Meghna (GBM) Basin, which covers nearly 1.75 million
km2 spread over five countries (Bangladesh, Bhutan, China, India and Nepal).
Located in the tropical monsoon belt, the LME is strongly affected by monsoons, storm
surges, cyclones and tsunamis. During the northeast monsoon, an anticyclonic gyre
forms in the Bay and reverses during the southwest monsoon (Wyrtki 1973, Longhurst
1998). The LME shows considerable spatial and temporal variability because of
seasonal river discharges, particularly the surface water along the coast. Monsoon rain
and flood waters produce a warm, low-salinity, nutrient and oxygen-rich layer to a depth
of 100 - 150 m; this layer floats above a deeper, more saline, cooler layer that does not
change significantly with the monsoons (Dwivedi & Choubey 1998). Large quantities of
fresh water and sediment discharged into the LME have also contributed to the formation
of the largest mangrove system in the world, the Sunderbans, covering an area of 12,000
km2 and shared by India and Bangladesh. Books and book chapters, reports and articles
pertaining to this LME include Dwivedi (1993), Aziz et al. (1998), Desai & Bhargava
(1998), Dwivedi & Choubey (1998), Ittekot et al. (2003), Silvestre and Pauly (1997),
Silvestre et al. (2003) and UNEP (2006).
I. Productivity
The Bay of Bengal LME can be considered a Class I, highly productive ecosystem (>300
gCm-2yr-1).While large nutrient input from river run-off supports high primary production in
coastal waters, the central parts of the bay are less productive because of the absence of
large-scale mixing or upwelling (Dwivedi 1993). The presence of different water masses
in coastal areas has produced sub-systems along the coast that differ in their
environmental characteristics and community composition. These sub-systems are
described by Dwivedi (1993). Secondary production is highest in the post-monsoon
period (October to January) and lowest during the monsoon period from June to
September (Desai & Bhargava 1998). Zooplankton biomass is low near the shore but
increases towards the EEZ boundary (Desai & Bhargava 1998). Further information on
biological production and fishery potential in India's EEZ is given in Desai & Bhargava
(1998). Wetlands, marshes, mangroves, backwaters and coastal lakes play an important
role in overall productivity (Dwivedi 1993). The coastal forested areas of Sri Lanka and
Malaysia are biodiversity hotspots, with a large number of threatened endemic plants and
animals (Aziz et al. 1998).
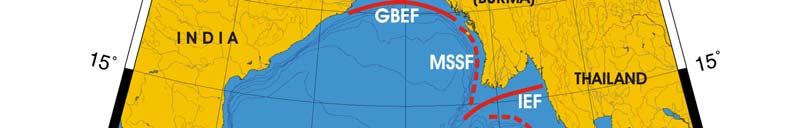
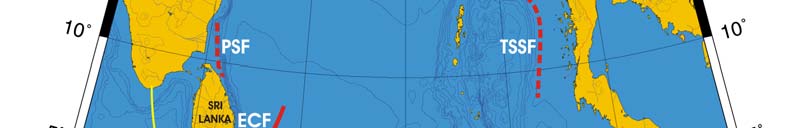
Oceanic fronts (after Belkin et al. (2008)): The principal front in the Bay of Bengal is
maintained by the huge fresh outflow from the Ganges-Brahmaputra estuary (Figure VII-
10.1). This is a year-round front, whose cross-frontal TS-ranges vary seasonally.
Another estuarine front is maintained by the Irravadi River outflow in the northern
238
10. Bay of Bengal LME
Andaman Sea. In both cases the location of estuarine fronts coincides with the shelf
break. A front east of Sri Lanka has been recently described from satellite data (Belkin et
al. 2005); its origin is related to the wind-induced upwelling off the east coast of Sri
Lanka. A bathymetrically-trapped front exists along a sill at the northern entrance to the
Palk Strait between India and Sri Lanka.
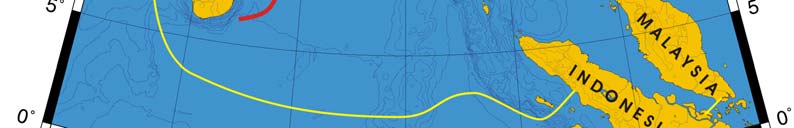
Figure VII-10.1. Fronts of the Bay of Bengal LME. ECF, East Ceylon Front; GBEF, Ganges-Brahmaputra
Estuarine Front; IEF, Irravadi Estuarine Front; MSSF, Myanmar Shelf-Slope Front; PSF, Palk Strait
Front; TSSF, Thailand Shelf-Slope Front. Red dashed lines, most probable locations of fronts. Yellow
line, LME boundary. Belkin et al. (2008).
Bay of Bengal SST (after Belkin 2008)
Linear SST trend since 1957: 0.50°C.
Linear SST trend since 1982: 0.24°C.
The steady, slow warming of the Bay of Bengal was modulated by quasi-regular
interannual variability with an average magnitude of <0.5°C. The dominant mode of
variability has a scale of 3 to 5 years, whereas decadal variability is not distinct. The all-
time maximum of 1998 occurred simultaneously with other Indian Ocean LMEs and could
be linked to El Niņo 1997-1998. It is more difficult to correlate other extrema with similar
events elsewhere since the Bay of Bengal LME has no immediate LME neighbors. For
example, the all-time minimum of 1961 has no contemporary counterparts elsewhere in
the Indian Ocean and therefore must be explained locally.
The temperature history of the Bay of Bengal is strongly coupled with its salinity regime,
since the upper layer stability here is largely dependent on the freshwater discharge of
VII South Asian Seas
239
three great rivers, the Ganges, Brahmaputra and Irrawaddy. The river discharge is
seasonal to the extreme, governed by the Indian monsoon, which brings heavy
precipitation to the Indian subcontinent (e.g. Salahuddin et al. 2006). Therefore
interannual variability of the Indian monsoon largely determines the river discharge,
hence salinity regime and eventually SST variability, in the Bay of Bengal. The Bay of
Bengal is not spatially uniform, notwithstanding the existence of a quasi-stationary gyre
circulation encompassing the Bay. The horizontal non-uniformity is caused by the
perennially low salinity in the northern Bay owing to the Ganges-Brahmaputra river
discharge. As a result, the upper mixed layer in the northern Bay is much shallower than
in the south. The boundary between these two regimes runs zonally along ~15°N
(Narvekar and Kumar 2006). This separation of the Bay of Bengal into two parts,
northern and southern, with different SST regimes, must have important ecosystem
ramifications.
Figure VII-10.2. Bay of Bengal LME annual mean SST (left) and SST anomalies (right), 1957-2006, based
on Hadley climatology. After Belkin (2008).
Bay of Bengal LME trends in Chlorophyll and Primary Productivity: The Bay of
Bengal LME can be considered a Class I, highly productive ecosystem (>300 gCm-2yr-1).
Figure VII-10-3. Bay of Bengal LME annual trends in chlorophyll a (left) and primary productivity (right),
1998-2006. Values are colour coded to the right hand ordinate. Figure courtesy of J. O'Reilly and K.
Hyde. Sources discussed p. 15 this volume.
240
10. Bay of Bengal LME
II. Fish and Fisheries
Fisheries of the Bay of Bengal LME target a wide range of species, including sardine,
anchovy, scad, shad, mackerel, snapper, emperor, grouper, pike-eel, tuna, shark,
ornamental reef fish, shrimp, bivalve shellfish and seaweed (Preston 2004). Catches
from commercial and subsistence fishing equal or exceed those from industrial fisheries.
In Bangladesh, for example, less than 5% of marine landings are estimated to come from
industrial fishing, with the rest coming from the artisanal sector (Hossain 2003;
Chuenpagdee et al. 2006). During the last decade, some countries have developed
offshore fishing for tuna, notably Indonesia, Thailand and Sri Lanka and while most of the
tuna catch comes from coastal fisheries, offshore fisheries provide the majority of export-
grade tuna (Preston 2004). Crustacean catch is slightly less than 15% of the total catch,
with penaeid shrimp accounting for about 40% of the total crustacean catch and being
the major export earner (FAO 2003). Most of the countries are also major producers of
farmed shrimps, with Thailand and Indonesia among the world's top producers (FAO
2005a).
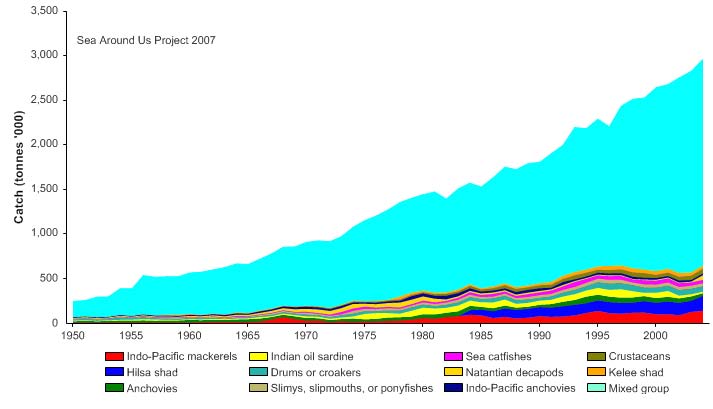
Statistics on fisheries catch and effort are highly fragmented, especially in the artisanal
and subsistence fisheries, two very important sectors in the region (Preston 2004). There
are also indications that a continuous increase in the reported landings, particularly of
unidentified fishes (included in `mixed group' in Figure VII-10.4), may be a product of
deficiencies in the underlying statistics, rather than improvements in the performance of
the fisheries in the LME (Figure VII-10.4. If so, such deficiencies would have serious
implications on the effectiveness of the fisheries management regimes in the LME and
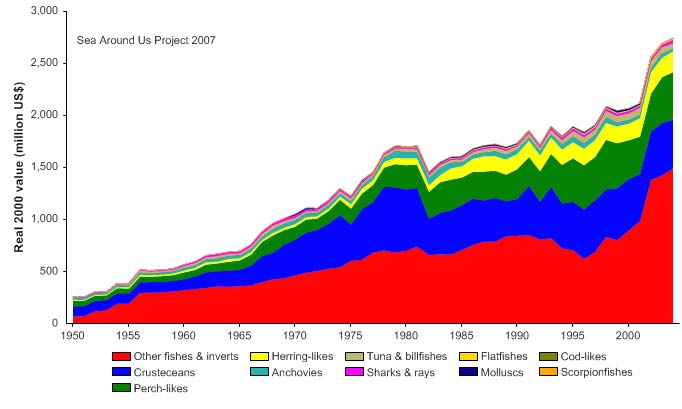
would also affect the value of the reported landings, which, according to Figure VII-10.5,
rose to about over 2.7 billion US$ (in 2000 real US$) in 2004.
Figure VII-10.4. Total reported landings in the Bay of Bengal LME by species (Sea Around Us 2007).
VII South Asian Seas
241
Figure VII-10.5. Value of reported landings in the Bay of Bengal LME by commercial groups (Sea
Around Us 2007).
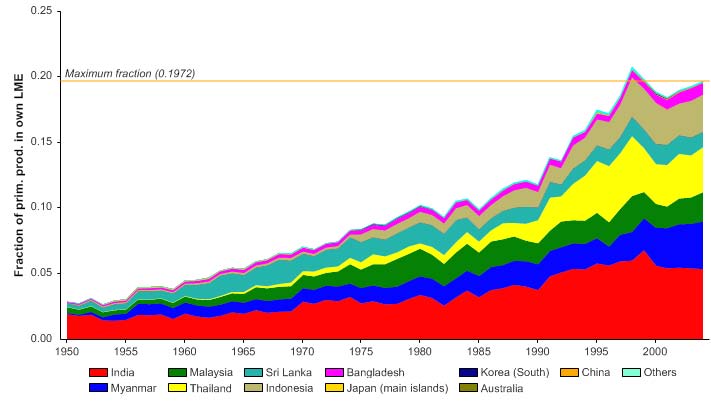
The primary production required (PPR; Pauly & Christensen 1995) to sustain the reported
landings in this LME has increased over the years, and reached 20% of the observed
primary production in 1998 (Figure VII-10.6). Such high PPR is another indication that
the reported landings for this LME may be exaggerated. Bordering countries, namely
India, Myanmar, Malaysia and Thailand account for the largest shares of the ecological
footprint in the region.
Figure VII-10.6. Primary production required to support reported landings (i.e., ecological footprint) as
fraction of the observed primary production in the Bay of Bengal LME (Sea Around Us 2007). The
`Maximum fraction' denotes the mean of the 5 highest values.
The mean trophic level of the reported landings (i.e., the MTI; Pauly & Watson 2005)
show a steady decline over the past 50 years (Figure VII-10.7 top) while the FiB index
increased over the same period (Figure VII-10.7 bottom). Due to the nature of the
242
10. Bay of Bengal LME
underlying landings statistics, it is not possible to draw any reasonable conclusions from
these indices, however, a detailed analysis of the MTI and FiB index of Western India,
based on independently validated catch data from the States and Union Territories
(Bhathal 2005), found that a `fishing down' of the food webs (Pauly et al. 1998) is indeed
occurring in the region (Bhatal and Pauly, in press).
Figure VII-10.7. Mean trophic level (i.e., Marine Trophic Index) (top) and Fishing-in-Balance Index
(bottom) in the Bay of Bengal LME (Sea Around Us 2007).
The Stock-Catch Status Plots indicate that the number of collapsed and overexploited
stocks in the LME is low but on the rise (Figure VII-10.8, top), with over 80% of the
reported landings from fully exploited stocks (Figure VII-10.8, bottom). Again, the
questionable quality of the underlying landings statistics must be noted.
As should be expected, given the amount of fishing pressure present in this LME (Gelchu
and Pauly 2007), both the catch per unit effort and the average size and weight of the
catches have been on a decline (Preston 2004). Excess fishing capacity in many of the
region's coastal fisheries is reducing the productivity of the local stocks and threatening
their long-term sustainability (Preston 2004). In fact, intensive fishing has been identified
as the primary force driving biomass changes in the LME (Sherman 2003). These
changes are well illustrated on the southeast coast of India, where high density of coastal
fishing craft is inducing changes in the ecosystem, as evident in the trophic level declines
(Bhathal 2005, Vivekanandan et al. 2005). India, for example, is experiencing serial
depletions of coastal fish stocks, where the increase in its fisheries catch is maintained
only by the expansion of its range. Indeed, there are now signs that this expansion
phase has reached its limit, with stagnation of its catch (Bhathal 2005). Other indicators
of unsustainable resource use are described in the Bay of Bengal LME national reports
for a wide range of resources including finfish, shark, crustacean, mollusc and
echinoderm (Preston 2004).
Destructive fishing practices of various kinds are commonplace in the LME. Continued
growth of commercial fishing effort, especially by trawlers, is increasing the fishing
mortality of non-reef species. In the southern Indian maritime states of Tamil Nadu and
VII South Asian Seas
243
Andhra Pradesh, the decline in the catch has been associated with an increase in
unregulated trawling for shrimps.
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
)
80
%
(
30%
t
us
70
t
a
s
40%
60
by
s
k
50%
50
t
oc
s
60%
40
r
of
e
b
70%
m
30
u
N
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 6022)
developing
fully exploited
over-exploited
collapsed
1950
1960
1970
1980
1990
2000
0%
100
10%
90
20%
80
)
%
30%
(
70
s
u
at
40%
60
ck st
50%
o
50
st
y
60%
b
h
40
t
c
70%
Ca
30
80%
20
90%
10
100%0
1950
1960
1970
1980
1990
2000
(n = 6022)
developing
fully exploited
over-exploited
collapsed
Figure VII-10.8. Stock-Catch Status Plots for the Bay of Bengal LME, showing the proportion of
developing (green), fully exploited (yellow), overexploited (orange) and collapsed (purple) fisheries by
number of stocks (top) and by catch biomass (bottom) from 1950 to 2004. Note that (n), the number of
`stocks', i.e., individual landings time series, only include taxonomic entities at species, genus or family
level, i.e., higher and pooled groups have been excluded (see Pauly et al, this vol. for definitions).
Excessive bycatch is of concern, although all captured fish are generally used either for
human consumption or as aquaculture feed. The accidental capture of endangered fish
species, dolphin and sea turtle is also of concern. The large-scale collection of fish and
shrimp larvae for aquaculture using destructive methods may be seriously damaging wild
stocks of both shrimp and other species (FAO 2005a), which typically make up more than
99% of the catch (Preston 2004). Dynamite fishing, often for small pelagic species, and
the use of cyanide and other toxins for capturing ornamental and live food fish, are both
increasing, and may lead to long-term damage, not only to the target resources, but to
their associated habitats (FAO 2002, Preston 2004).
Expanding human populations of the Bay of Bengal LME region has created an
increasing demand for fish as a source of animal protein. Furthermore, trade
liberalisation and rising demand for export have contributed to the rapid development of
marine fisheries and aquaculture in recent years. The steady decline in the abundance
of the fisheries resources is expected to continue, despite a number of regulatory
244
10. Bay of Bengal LME
measures in force in some of the bordering countries. Bilateral or multilateral
collaboration would greatly assist the efforts of individual countries in addressing the
problem of overexploitation, given the transboundary nature of most of the fish stocks.
III. Pollution and Ecosystem Health
Pollution: Human activities are causing serious environmental degradation, threatening
the sustainable management and health of the near-coastal waters. Among the major
threats to the LME's health and productivity is pollution from land-based sources,
particularly related to sewage, agriculture, aquaculture and industries (Kaly 2004,
Samarakoon 2006). These are also the main land-based pollution categories of
transboundary significance in the region. The mobilisation of pol utants through rivers,
run-off and floods, as well as cross-border movements of pollutants through international
rivers, are of concern (Kaly 2004). Pollution from sea-based sources (oil spills, oil
exploration and production) is also among the main recognised threats (Kaly 2004).
Sewage was identified as a major priority issue (Chia & Kirkman 2000). This includes
nutrients, POPs, household chemicals, medical wastes, excreted pharmaceuticals and
sediments. The use of chemicals and irrigation in agriculture and aquaculture, as well as
sediment inputs to the coastal areas compounds this problem. High amounts of organic
and inorganic nutrients reach the LME (Kaly 2004). Although the ecological effect of
nutrient enrichment of the coastal environment of the LME are poorly documented and
understood, reported localised problems of eutrophication, hypoxia and algal blooms are
likely to be related. Over the past 20-30 years, an increase in both the frequency and
persistence of algal blooms in coastal waters and enclosed sea areas in India has been
reported (Sampath 2003). The GBM river system is a major recipient of waste from
industries in Bangladesh and India. High levels of pesticides can be found along the
coast, especially near cities and ports (Dwivedi 1993).
Pollution by suspended solids is common to the entire LME, including the Andaman Sea.
Although sediment mobilisation occurs with urban and port developments, the most
important sources are probably deforestation together with agriculture and aquaculture
(Kaly 2004). The GBM river system delivers 30% of the world's total load of river
sediment (Milliman & Meade 1983), and provide high turbidity in the coastal waters, as
has been shown in satellite photos.
Oil spills are a major concern. There is heavy oil tanker traffic between Japan and the
Middle East, with the main shipping route passing south of Sri Lanka before entering the
Straits of Malacca. Along the Indian coastline, there is also intense shipping traffic, and
associated chronic oil pollution through operational discharge of waste, mostly by
medium and small ships where installation of oil-water separators is not mandatory
(Sampath 2003). Increasing shipping activity and increasing emphasis on offshore oil
exploration in many countries of the region makes the northern Indian Ocean very
vulnerable to oil pollution.
Habitat and community modification: Among the coastal habitats of the Bay of Bengal
LME are several wetlands of international importance (WRI 2005). Six areas of critical
biological diversity are the Sundarbans, Palk Bay and the Gulf of Mannar, the Marine
(Wandur) National Park in the Andaman and Nicobar Islands, the Maldives Atolls, Mu Ko
Similan National Park and Mu Ko Surin National Park in Thailand. The Sundarbans, a
UNESCO World Heritage Site, represents the most economically important production
forest and natural wildlife habitat in Bangladesh.
Extensive habitat modification has occurred, but was considered to be moderate in
Bangladesh, India, and Sri Lanka, and severe in the Andaman Sea. The major problems
VII South Asian Seas
245
are sedimentation and siltation, reclamation, coastal aquaculture, illegal fishing, and oil
pollution, as well as global warming and sea level rise (Angell 2004). Climate change is
likely to have severe impacts on the LME as it is closed in the north, preventing the
migration of endemic species to higher latitudes. The impact on the ecosystem of the
recent start of dredging in the Gulf of Mannar for the Sethusamudram Ship Canal is also
of grave concern.
Weakened traditional common property management, growing human population in
coastal areas, and development of brackish water shrimp farming have contributed to the
increasing pressure on mangrove forests and their resources in the last few decades
(Angell 2004, Samarakoon 2004). With a few exceptions, most mangrove habitats in the
Bay of Bengal LME region are degraded or threatened. For instance, in the Sundarbans,
some 150,000 ha of mangrove forest disappeared during the past 100 years, as a result
of reclamation for agriculture settlement sites, industrial estates and roads (Govindasamy
et al. 1997). More than half of the total area (some 208,220 ha) of Thailand's mangrove
forests disappeared between 1961 and 1993 (GESAMP 1993). Between 1991 and 1995,
approximately 50,000 ha of coastal wetlands along the east coast of India were
converted to shrimp farms (Government of India 2002). In Sri Lanka, mangrove
conversion to shrimp ponds has considerably reduced mangrove forest (Joseph 2003).
Agriculture and land reclamation for urban settlements have also reduced the mangroves
and peat swamps of the Malacca Straits by about 50-60% (Thia-Eng et al. 1997).
Similarly, the Merbok mangroves in Malaysia, with one of the highest recorded levels of
species diversity in the world, have been reduced by about 65% through conversion to
rice paddies, shrimp farms and housing estates (Samarakoon 2004).
Among the pressures on the region's coral reefs are destructive fishing practices, siltation
and pollution, unplanned tourism development and coral mining (Angell 2004) are
prominent. Coral reefs have also been damaged by bleaching, as a consequence of
periodic increases in sea surface temperatures. The most notable bleaching event
occurred in 1997-1998, and caused extensive bleaching and in numerous instances, over
90% mortality of corals, in some parts of the LME (Wafar 1999, Chou et al. 2002,
Wilkinson 2002). Pollution and related disease are also threatening some reefs. For
instance, oil spills and ballast water discharges are a significant threat to 85% of
Thailand's reefs (Angell 2004). Destructive fishing practices such as the use of cyanide
and explosives are a major cause of coral reef degradation in most of the countries,
particularly in Indonesia, where 67%-98% of the reefs are seriously degraded.
Furthermore, reefs are generally depleted of high value food fish due to the demand for
both the local tourism industry and export. Although this practice has been banned, coral
mining has destroyed coral reefs in many areas, including in Sri Lanka, India and, to a
lesser extent, in Bangladesh. Only the Maldives government has had some success in
reducing this destructive practice by subsidising the import of alternative materials.
Extensive damage to coastal and marine habitats was caused by the tsunami of 26
December 2004 (CORDIO 2005a, 2005b, IUCN/CORDIO 2005). Places along the coast
that were most affected were those that have been previously disturbed by anthropogenic
activities. For example, mangroves and vegetated coastal dunes seem to have
dissipated the wave energy and provided protection to coastlines, coastal inhabitants and
infrastructure. Surveys have shown significant damage to coral reefs over extensive
areas from mechanical damage, deposition of debris, sand, silt, and rubble, as well as
impacts on the diversity of benthic organisms and fish. Fish populations, which in many
cases were depleted by overexploitation, showed varying levels of impact, seemingly
correlated with loss of habitat. In general, a higher impact was observed on smaller fish,
notably damselfish, gobies, butterfly fish and wrasse; this may have adverse
consequences for the ornamental fish trade.
246
10. Bay of Bengal LME
The impact of the tsunami is also very visible on turtle nesting sites (Kulkarni 2005,
CORDIO 2005b). The nesting beaches of leatherback, green, hawksbill and olive ridley
turtles in South Andaman, Little Andaman and the Nicobar Group of islands have almost
vanished. Sand and sediment deposited on sea grass beds will have a long term-impact
on dugongs, which feed in these areas. Severe beach erosion has occurred at all sites,
with some beaches suffering over 50% reduction in width and up to one meter loss in
height.
IV. Socioeconomic Conditions
The eight countries bordering the Bay of Bengal LME include some of the most populous
in the world, with India, Indonesia and Bangladesh being among the world's top ten. An
estimated 400 million people live in the LME's catchment area (Preston 2004). The LME
and its natural resources are of considerable social and economic importance to the
bordering countries, with activities such as fishing, shrimp farming, tourism and shipping
contributing to food security, livelihoods, employment and national economies. Marine
fisheries make a modest contribution to the GDP of the bordering countries, with the
exception of the Maldives, where this sector contributes 11% to GDP and 74% of the
country's export commodities (FAO 2005a). Primary export commodities are shrimp and
tuna, which make a significant contribution to national foreign exchange earnings. For
example, in Bangladesh, fisheries account for more than 11% of annual export earnings,
while in Indonesia, the value of fisheries exports amounted to about US$1.6 billion in
1998 (FAO 2005a).
Rapid development of aquaculture, mainly of shrimp, in the extensive coastal and
brackish-water areas has made a significant contribution to the growth of national export
earnings, and aquaculture is now an important element in both the local and national
economies. Based on statistics in FAO (2005b), the combined output of the region's
farmed shrimp and fish in 2003 was estimated at about 5.3 million tonnes, equivalent to
35% of total production from capture and aquaculture. It should be noted, however, that
these statistics are based on the countries' total production, and not only that from the
LME, although most of the aquaculture production comes from the LME. Tourism also
makes a substantial contribution to the national economies of some of the Bay of Bengal
LME countries. Coastal tourism in western Thailand, Peninsular Malaysia, Sri Lanka and
the Maldives continue to gather momentum and is being promoted in India and
Bangladesh.
Many of the region's poor are dependent primarily or entirely on marine resources, and
have few, if any alternatives to fishing, even when overfishing is clearly occurring
(Preston 2004, Samarakoon 2004). Fisheries also provide employment for millions of
people. For example, in Indonesia, over 5 million people are directly involved in fishing
and fish farming. Together with their families, they make up at least 4 percent of the total
population (FAO 2005a). In Bangladesh, this sector provides income to some 1.5 to
2 million full-time and around 12 million part-time fishers, while in the Maldives, fisheries
account for 20% of employment. Fisheries also make a very important contribution to the
national diet in the bordering countries (FAO 2005a). For example, about two-thirds of
Bangladesh, Indonesia and Sri Lanka national protein supply come from fish. This is
even higher in Mayanmar, where fish makes up 80% of the animal protein for most
people.
The socioeconomic impacts of over-exploitation were assessed as severe in the Bay of
Bengal LME countries, particularly for the millions of poor coastal fisher families.
Increasing fishing effort and declining resources are leading to increased competition for
access to these resources, with negative impacts, especially on poorer resource users
(Townsley 2004). Reduced benefit flows from resource use lead to reduced livelihood
VII South Asian Seas
247
security, including reduced food security. The localised decline of fisheries resources
also forces resource users to migrate to other areas in search of new opportunities. This
creates new vulnerabilities for those affected as it means abandoning familiar
environments and social support networks. Without the capacity to adopt alternative
strategies, poorer groups continue to exploit fisheries resources, further exacerbating the
decline of the resources (Townsley 2004).
Pollution is affecting both critical habitats in coastal and marine areas, and the livelihoods
that depend on them. Those making direct use of these resources see decreasing
access to resources, declining environmental conditions that may affect their access to
safe water and necessary livelihood resources and specific health risks generated by
increased pol ution (Townsley 2004). Over 60% of reported diseases in the two countries
are linked to pollution discharged from point and diffuse sources. Pollution impacts are
often particularly severe in coastal areas where pollution from multiple sources may be
concentrated.
The coastal and marine habitats of the Bay of Bengal LME serve as nursery areas for fish
and shellfish species that contribute substantially to income, livelihood, food security and
employment in the bordering countries. These benefits are lost or threatened when such
habitats are destroyed. The extent to which this affects other countries around the LME
is unclear, but the interconnectedness of marine ecotones suggests that there are likely
to be impacts, particularly in adjacent areas but also potentially further away (Townsley
2004; Bhattacharya and Sarkar 2003). For instance, distant fisheries may be affected by
the destruction of habitats that are critical to the life cycle of their target species.
Many of the marine and coastal environmental problems faced by the Bay of Bengal LME
are inextricably linked with the large populations of the region's coastal areas, and their
impoverished status. Continued population growth, and the increasing concentration of
people in coastal areas will exacerbate these problems in the future. Unless addressed,
environmental degradation and unsustainable resource use practices will reduce the
capacity of fisheries to provide sustenance and income for coastal people, thus leading to
increased poverty in a spiralling effect. Preston (2004) notes the growing need to
address coastal management, pollution, fishery management and alternative livelihood
issues in parallel.
V. Governance
The LME is bordered by Bangladesh, India, Indonesia, Malaysia, Maldives, Myanmar, Sri
Lanka and Thailand. At the national level, a range of environmental and fisheries
regulations and management initiatives have been developed by the countries bordering
the LME (Edeson 2004, FAO 2005a). However, their results have been mixed, with
effectiveness hampered largely by inadequate implementation, surveillance and
enforcement. Attempts to conserve coral reefs focus on the establishment of MPAs.
These may be internationally recognised biosphere reserves or nationally established
marine protected areas or parks (Angell 2004). For instance, the Sundarbans and the
Gulf of Manner were named biosphere reserves in 1986 and are recognised by UNESCO
under their `Man in the Biosphere' programme. The effectiveness of these MPAs,
however, varies considerably. Problems include intrusion of local fishers, weak to non-
enforcement of MPA regulations, and lack of coordination among responsible
government agencies.
A multitude of international, regional, and sub-regional organisations and programmes
operate in the Bay of Bengal LME. The only regional fisheries management organisation
whose jurisdiction extends into the LME is the Indian Ocean Tuna Commission. There
are also numerous stakeholder groups and policy frameworks (Aziz et al. 1998). In March
248
10. Bay of Bengal LME
1995, the South Asian Seas Action Plan (SASAP) was adopted by Bangladesh, India,
Maldives, Pakistan and Sri Lanka. The South Asia Cooperative Environment Programme
is the Action Plan secretariat. Although there is not yet a regional convention, SASAP
follows existing global environmental and maritime conventions and considers the Law of
the Sea as its umbrella convention. One of SASAP's priorities focuses on National
Action Plans and pilot programmes to implement the GPA.
The regional Bay of Bengal Programme (BOBP) started out in 1979 as a fisheries
development oriented-programme, and moved progressively towards fisheries
management. The BOBP has been succeeded, in a reduced form, by the Bay of Bengal
Programme Inter-Governmental Organisation, which continues to promote responsible
management of small-scale fisheries and related activities. This organization has a
membership of Maldives, India, Sri Lanka and Bangladesh and focuses largely on coastal
fisheries related issues of these countries.
Recognising the need for integrated and coordinated management of their coastal and
near-shore living marine resources, the eight countries bordering the LME have
embarked on the development of a Bay of Bengal Large Marine Ecosystem Project with
support from GEF to address critical threats to the coastal and marine environment, and
to promote ecosystem-based management of the LME's coastal and marine resources.
This project has recently been endorsed by GEF and will be implemented 2008-2013 by
FAO with the aim of increased national institutional capacity in participating countries.
Through this process, the outcomes will be a Trans-boundary Diagnostic Analysis,
including assessments of critical coastal/marine habitats providing a location-specific
assessment of critical transboundary concerns and the identification of "hotspots". As a
part of regional cooperative arrangements, a permanent, partially financially-sustainable
institutional arrangement will be established, that will support the continued development
and broadening of commitment to a regional approach to BOBLME issues. The Strategic
Action Plan that will be developed will guide future BOBLME Programme activities
leading to improved wellbeing of rural fisher communities through incorporating regional
approaches to resolving resource issues and barriers affecting their livelihoods.
The BOBLME will be largely based around regional and sub-regional activities for
collaborative ecosystem approaches leading to changes in sources and underlying
causal agents contributing to trans-boundary environmental degradation. The
programme also envisages action to promote the restoration of depleted stocks and
develop a better understanding of the BOBLME's large-scale processes and ecological
dynamics. Basic health indicators in the BOBLME will be established as part of this. As
a goal over the longer-term, and foreseen within the Strategic Action Plan, the sustained
commitment from the BOBLME countries to collaborate will be achieved through adoption
of an agreed institutional collaborative mechanism.
References
Angell, C.L. (2004). Review of Critical Habitats- Mangroves and Coral Reefs. Bay of Bengal Large
Marine Ecosystem (BOBLME) Theme report, FAO-BOBLME Programme.
Aziz, A.H., Ahmed, L., Atapattu, A., Chullasorn, S., Pong Lui, Y., Maniku, H.M., Nickerson, D.J.,
Pimoljinda, J., Purwaka, T.H., Saeed, S., Soetopo, S., and Yadava, Y.S. (1998). Regional
stewardship for sustainable marine resources management in the Bay of Bengal, p 369-378 in:
K. Sherman, E. Okemwa and M. Ntiba (eds), Large Marine Ecosystems of the Indian Ocean:
Assessment, Sustainability, and Management. Blackwell Science.
Bhattacharya, A. and Sarkar, S.K. (2003) Impact of overexploitation of shellfish: Northeastern
coast of India. Ambio 32(1) 70-75.
VII South Asian Seas
249
Belkin, I.M. (2008) Rapid warming of Large Marine Ecosystems, Progress in Oceanography, in
press.
Belkin, I.M., Cornillon, P.C., and Sherman, K. (2008). Fronts in Large Marine Ecosystems of the
world's oceans: An atlas. Progress in Oceanography, in press.
Bhathal, B. (2005). Historical reconstruction of Indian marine fisheries catches, 1950-2000, as a
basis for testing the `Marine Trophic Index'. Fisheries Centre Research Reports 13(4), 122 p.
Bhathal, B. and Pauly, D. (in press). `Fishing down marine food webs' and spatial expansion of
coastal fisheries in India, 1950-2000. Fisheries Research
Chia, L.S. and Kirkman, H. (2000). Overview of Land-Based Sources and Activities Affecting the
Marine Environment in the East Asian Seas. UNEP/GPA Coordination Office and EAS/RCU
(2000). Regional Seas Report and Studies Series.
Chou, L. M., Tuan, V.S., Philreefs, Yeemin, T., Cabanban, A., Suharsono and Kessna, I. (2002).
Status of Southeast Asia coral reefs, p 123-152 in: Wilkinson, C.R. (ed), Status of Coral Reefs
of the World 2002. GCRMN Report, Australian Institute of Marine Science, Townsville,
Australia.
Chuenpagdee, R., Liguori, L., Palomares, M.L.D. and Pauly, D. (2006). Bottom-up, Global
Estimates of Small-Scale Marine Fisheries Catches. Fisheries Centre Research Reports 14(8).
Fisheries Centre, University of British Columbia, Vancouver, Canada, 105 pp.
CORDIO (2005a). Assessment of tsunami damage in the Indian Ocean: First Report CORDIO
online document. www.cordio.org/news_article.asp?id=11
CORDIO (2005b). Assessment of tsunami damage in the Indian Ocean: Second Report CORDIO
online document. www.cordio.org/news_article.asp?id=12
Desai, B.N. and Bhargava, R.M.S. (1998). Biologic production and fishery potential of the Exclusive
Economic Zone of India, p 297-309 in: Sherman, K., Okemwa, E. and Ntiba, M. (eds), Large
Marine Ecosystems of the Indian Ocean: Assessment, Sustainability, and Management.
Blackwell Science Inc. Cambridge, MA.
Dwivedi, S.N. (1993). Long-term variability in the food chains, biomass yield and oceanography of
the Bay of Bengal Ecosystem, p 43-52 in: Sherman, K., Alexander, L.M. and Gold, B.D. (eds),
Large Marine Ecosystems: Stress, Mitigation, and Sustainability. AAAS Press. Washington,
D.C.
Dwivedi, S.N. and Choubey, A.K. (1998). Indian Ocean Large Marine Ecosystems: Need for
National and Regional Framework for Conservation and Sustainable Development, p 361-368
in: K. Sherman, E. Okemwa and M. Ntiba (eds), Large Marine Ecosystems of the Indian Ocean:
Assessment, Sustainability, and Management. Blackwell Science Inc. Cambridge, MA.
Edeson, W. (2004). Review of Legal and Enforcement Mechanisms in the BOBLME Region. Bay of
Bengal Large Marine Ecosystem (BOBLME) Theme report. GCP/RAS/179/WBG. FAO-
BOBLME Programme.
FAO (2002). The State of World Fisheries and Aquaculture. Food and Agriculture Organisation of
the United Nations, Rome, Italy.
FAO (2003). Trends in Oceanic Captures and Clustering of Large Marine Ecosystems - 2 Studies
based on the FAO Capture Database. FAO Fisheries Technical Paper 435.
FAO (2005a). Fishery Country Profiles at.www.fao.org/fi/fcp/fcp.asp.
FAO (2005b). World fisheries production, by capture and aquaculture, by country (2003). FAO
Yearbook of Fisheries Statistics: Summary Tables 2003. www.fao.org/fi/statist/statist.asp
FAO (2007) Bay of Bengal LME Prospectus, www.fao.org/fi/boblme/website/index.htm
Gelchu, A. and Pauly, D. (2007). Growth and distribution of part-based fishing effort within
countries' EEZ from 1970 to 1995. Fisheries Centre Research Reports, 15(4), 99 p.
GESAMP (1993). Joint group of experts on the scientific aspects of marine protection, impact of oil
and related chemicals and wastes on the marine environment. Reports and Studies 50. IMO,
London, U.K.
Government of India (2002). National Biodiversity Action Plan for the East Coast of India.
http://sdnp.dehi.nic/nbsap/dactionp/ecoregion/ecoast/ecoast.html
Govindasamy C., Viji Roy, A.G., Prabhahar, C., Valarmathi, S. and Azariah, J. (1997). Bioethics in
India, in: Azariah, J., Azariah, H. and Macer, D.R.J. (eds), Proceedings of the International
Bioethics Workshop in Madras: Biomanagement of Biogeoresources, 16-19 Jan. 1997,
University of Madras.
Hossain, M.M.M. (2003). National Report of Bangladesh on the Sustainable Management of the
Bay of Bengal Large Marine Ecosystem. Bay of Bengal LME Project, FAO GCP/ RAS/ 179/
WBG, Chennai, India.
Ittekot, V., Kudrass, H.R., Quaddfasel, D. and Unger, D. (eds.) (2003). The Bay of Bengal. Deep
Sea Research, Part II:Topical Studies in Oceanography. 50(5): 853-1045.
250
10. Bay of Bengal LME
IUCN/CORDIO (2005). Initial Rapid Assessment of Tsunami Damage to Coral Reefs in Eastern Sri
Lanka, 2-5 March 2005. www.cordio.org/news_article.asp?id=20
Joseph, L. (2003). National Report of Sri Lanka on the Formulation of a Transboundary Diagnostic
Analysis and Strategic Action Plan for the Bay of Bengal Large Marine Ecosystem Programme.
BOBLME Report. FAO-BOBLME Programme
Kaly, U.L. (2004). Review of Land-based sources of pollution to the coastal and marine
environments in the BOBLME Region. Bay of Bengal Large Marine Ecosystem Theme Report.
FAO-BOBLME Programme.
Kulkarni, S. (2005). Tsunami Impact Assessment of Coral Reefs in the Andaman and Nicobar.
CORDIO online document. www.cordio.org/news_article.asp?id=13.
Longhurst, A.R. (1998). Ecological Geography of the Sea. Academic Press, California, USA.
Milliman, J.D. and Meade, R.H. (1983). Worldwide delivery of river sediment to the oceans. Journal
of Geology 91:1-21.
Narvekar, J., and Kumar, S.P. (2006) Seasonal variability of the mixed layer in the central Bay of
Bengal and associated changes in nutrients and chlorophyll Deep-Sea Res. Part I, 53(5), 820-
835.
Pauly, D. and Christensen, V. (1995). Primary production required to sustain global fisheries.
Nature 374: 255-257.
Pauly, D. and Watson, R. (2005). Background and interpretation of the `Marine Trophic Index' as a
measure of biodiversity. Philosophical Transactions of the Royal Society: Biological Sciences
360: 415-423.
Pauly, D., Christensen, V., Dalsgaard, J., Froese R. and Torres, F.C. Jr. (1998). Fishing down
marine food webs. Science 279: 860-863.
Preston, G.L. (2004). Review of the Status of Shared/Common Marine Living Resource Stocks and
of Stock Assessment Capability in the BoBLME Region. Bay of Bengal Large Marine
Ecosystem (BOBLME) Theme report, FAO-BOBLME Programme.
Salahuddin, A., Isaac, R.H., Curtis, S. and Matsumoto, J. (2006) Teleconnections between the sea
surface temperature in the Bay of Bengal and monsoon rainfall in Bangladesh, Global and
Planetary Change, 53(3), 188-197.Samarakoon, J. (2004). Issues of livelihood, sustainable
development and governance: Bay of Bengal. Ambio 33(1-2): 34- 44.
Samarakoon, J. (2006). South Asian Seas Region, p 137 155 in: UNEP/GPA (2006), The State of
the Marine Environment: Regional Assessments. UNEP/GPA, The Hague.
Sampath, V. (2003). National Report on the Status and Development Potential of the Coastal and
Marine Environment of the East Coast of India and its Living Resources. BOBLME, FAO GCP/
RAS/ 179/ WBG, Chennai, India.
Sea Around Us (2007). A Global Database on Marine Fisheries and Ecosystems. Fisheries Centre,
University British Columbia, Vancouver, Canada. www.seaaroundus.org/lme/SummaryInfo
.aspx?LME=34
Sherman, K. (2003). Physical, biological, and human forcing of biomass yields in Large Marine
Ecosystems. ICES CM 2003/P:12.
Silvestre, G and Pauly, D. (eds). (1997). Status and Management of tropical coastal fisheries in
Asia. ICLARM Conference Proceedings 53, 208 p.
Silvestre, G.T, Garces, L.R., Stobutzki, I., Ahmed, M., Valmonte-Santos, R.A., Luna, C.Z., Lachica-
Aliņo, L., Munro, P., Christensen, V. and Pauly, D. (eds). (2003). Assessment, Management
and Future Directions for Coastal Fisheries in Asian Countries. WorldFish Center Conference
Proceedings 67, 1120 p. [Also available as CD-ROM]
Thia-Eng, C., Ross, S.A. and Hu, Y. (1997). Malacca Straits Environmental Profile.
GEF/UNDP/IMO Regional Programme for the Prevention and Management of Marine Pollution
in the East Asian Seas.
Townsley, P. (2004). Review of Coastal and Marine Livelihoods and Food Security in the Bay of
Bengal Large Marine Ecosystem Region. Report prepared for the Bay of Bengal Large Marine
Ecosystem Programme. FAO-BOBLME Programme.
Vivekanandan, E., Srinath, M. and Kuriakose, S. (2005). Fishing the marine food web along the
Indian coast. Fisheries Research 72 (2-3): 241-252.
Wafar, M.V.M. (1999). Status report India, p.25-26 in: Linden, O. and Sporrong, N. (eds), Coral
Reef Degradation in the Indian Ocean (CORDIO). Status Reports and Project Presentations
1999. Stockholm, Sweden.
Wilkinson, C. (2002). Coral bleaching and mortality - the 1998 event 4 years later and bleaching to
2002, p 33-44 in: Wilkinson, C.R. (ed), Status of Coral Reefs of the World: 2002. GCRMN
Report, Australian Institute of Marine Science, Townsville, Australia.
VII South Asian Seas
251
WRI (2005). Coastal and Marine Ecosystems: Country Profiles. http://earthtrends.wri.org/
country_profiles/index.cfm?theme=1&rcode=1
Wyrtki, K. (1973). Physical Oceanography of the Indian Ocean, p 18-36 in Zeitzschel, B. (ed),
Biology of the Indian Ocean. Springer-Verlag, Berlin.
252
10. Bay of Bengal LME







