




BCLME project LMR/CF/03/08
Report on BCLME project LMR/CF/03/08
Benguela Environment Fisheries I
nteraction & Training Programme
Review of the state of knowledge, research (past and present) of the
distribution, biology, ecology, and abundance of non-exploited mesopelagic
fish (Order Anguilliformes, Argentiniformes, Stomiiformes, Myctophiformes,
Aulopiformes) and the bearded goby (Sufflogobius bibarbatus) in the Benguela
Ecosystem.
A. Staby1 and J-O. Krakstad2
1 University of Bergen, Norway
2 Institute of Marine Research, Centre for Development Cooperation, Bergen, Norway
1
BCLME project LMR/CF/03/08
Table of Contents
1
Introduction................................................................................................................. 4
2
Materials and Methods................................................................................................ 7
2.1
Regional data sources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2.1.1
Nan-Sis........................................................................................................ 7
2.1.2
NatMIRC data............................................................................................. 8
3
Overview and Results ................................................................................................. 9
3.1
Gobies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
3.1.1
Species identity and diversity ..................................................................... 9
3.1.2
Species distribution..................................................................................... 9
3.1.3
Biology...................................................................................................... 15
3.1.4
Ecology ..................................................................................................... 16
3.1.5
Growth parameters.................................................................................... 17
3.1.6
Reproduction............................................................................................. 17
3.1.7
Abundance ................................................................................................ 18
3.1.8
Catch history ............................................................................................. 20
3.2
Mesopelagics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
3.2.1
Species identity and diversity ................................................................... 21
3.2.2
Species distribution................................................................................... 22
3.2.3
Biology...................................................................................................... 25
3.2.4
Ecology ..................................................................................................... 25
3.2.5
Life history................................................................................................ 26
3.2.6
Spawning and early life stages.................................................................. 28
3.2.7
Abundance ................................................................................................ 30
3.2.8
Catch history ............................................................................................. 31
3.3
Regional data sources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
3.3.1
Nan-Sis...................................................................................................... 32
3.3.2
MCM data ................................................................................................. 35
3.3.3
NatMIRC data........................................................................................... 35
3.4
Ongoing and recently completed research projects. . . . . . . . . . . . . . . . . . . . 37
4
Summary ................................................................................................................... 40
4.1
Recommendations on future studies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
5
References................................................................................................................. 42
5.1
Gobies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
5.2
Mesopelagics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
2
BCLME project LMR/CF/03/08
Annexes
Annex 1: Picture gallery of some mesopelagic species. . . . . . . . . . . . . . . . . . . . . . . 54
Annex 2: Picture gallery of some mesopelagic species . . . . . . . . . . . . . . . . . . . . . . . 55
Annex 3: Picture gallery of some mesopelagic species . . . . . . . . . . . . . . . . . . . . . . . 56
Annex 4: Nan-Sis database mesopelagic species listed - Angola. . . . . . . . . . . . . . . . 57
Annex 4: Nan-Sis database mesopelagic species listed Angola contnd.. . . . . . . . . 58
Annex 5: Nan-Sis database mesopelagic species listed - Namibia. . . . . . . . . . . . . . . 59
Annex 5: Nan-Sis database mesopelagic species listed Namibia contnd. . . . . . . . 60
Annex 6: Database mesopelagic species listed - South Africa. . . . . . . . . . . . . . . . . . 61
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 62
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 63
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 64
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 65
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 66
Annex 6: Database mesopelagic species listed - South Africa contnd.. . . . . . . . . . . 67
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT. . . . . . . . . . . . . 68
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT contnd. . . . . . . 69
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT contnd. . . . . . . 70
Annex 8: Distribution maps - Order Anguilliformes and Argentiformes. . . . . . . . . 71
Annex 9: Distribution maps - Order Stomiiformes. . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Annex 10: Distribution maps - Order Stomiiformes continued . . . . . . . . . . . . . . . . . 73
Annex 11: Distribution maps - Order Stomiiformes and Aulopiformes. . . . . . . . . . 74
Annex 12: Distribution maps - Order Myctophiformes. . . . . . . . . . . . . . . . . . . . . . . 75
Annex 13: Distribution of gobies and mesopelagic species collected by R.V.
Welwitchia during horse mackerel surveys. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Annex 14: Catch history . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Annex 15: BENFIT survey proposal on mesopelagics. . . . . . . . . . . . . . . . . . . . . . . . 78
Annex 16: Contact details. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84
3
BCLME project LMR/CF/03/08
1 Introduction
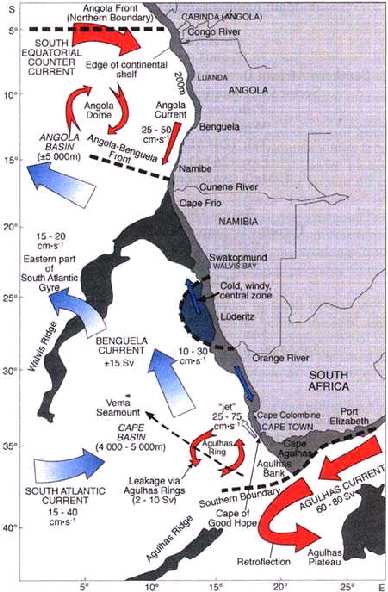
The Benguela upwelling system on the south west coast of Africa is one of the worlds'
four major western boundary upwelling regions, and one of the most productive marine
ecosystems in the world (Shannon, 1985). This marine system is characterised by a high
primary production rate due to strong upwelling occurring throughout the year and
peaking in late winter and spring. Essential nutrients are convected from the deeper
waters to the ocean surface as a result of this upwelling - the offshore movement of the
surface water masses, caused by strong southerly winds and the presence of colder deep
water masses (Garrison, 1998). The primary production is the basis for the large
production of pelagic fish of which the commercial species like sardine, anchovy, round
herring and horse mackerel has received most attention. However the most abundant
species are maybe the non-commercial mesopelagics and the gobies that play a key role
in the ecosystem, assimilating plankton and as prey making this energy available for
species higher in the food chain. The northern part of the ecosystem, the northern
Benguela, is also characterised by widespread areas of low oxygen waters overlying the
diatomaceous mud belt of the central Namibian shelf (Chapman and Shannon 1985,
Dingle and Nelson 1993), affecting the distribution of many demersal and pelagic
species. Gobies and some mesopelagic species seem to be among those species well
adapted to this hostile environment and are frequently found in these regions.
Figure 1: The Benguela Current Large Marine Ecosystem (BCLME). (Taken from Sumaila et al.
2003).
4
BCLME project LMR/CF/03/08
Gobies are reported to be the largest family of marine fishes (possibly > 2,000), and also
common in fresh and brackish waters. The smallest fishes (and vertebrates) in the world
belong to this family. Gobies are grouped together on the basis of several hard to discern
characters: bones of the head, and a family-unique sperm gland. Most live in or on the
bottom and are aptly adapted to a demersal existence. They are roughly torpedo-
cylindrically shaped, and have reduced lateral line systems coupled with enhanced vision.
Generally gobies lack swim-bladders and display degrees of fusion of their pelvic fins
that are located anteriorly under their pectorals and used as a sort of suction disc to help
them stay in place. Total length is usually reported to be < 10 cm but 50 cm maximum
length is known with some species. Most species are found in shallow coastal waters and
around coral reefs. The species are most commonly cryptic bottom dwelling carnivores of
small benthic invertebrates; others are planktivores. Some species have symbiotic
relationships with invertebrates (e.g. shrimps) and others are known to remove ecto-
parasites from other fishes. The gobies are typically nest spawners with non-spherical
eggs guarded by the male. Many are popular aquarium fishes. The following subfamilies
are recognized: Oxudercinae, Amblyopinae, Sicydiinae, Gobionellinae and Gobiinae
(Froese & Pauly 2005; Miller 1990; Nelson, 1994).
The Sufflogobius bibarbatus belongs to subfamily Gobiinae and is an abundant, presently
non-commercial species indigenous to the northern region of the Benguela ecosystem. It
is roughly confined to the coast of Namibia and the west coast of South Africa, but has
also been reported from the southern Angola (Figure 1 and 2, Nansen program,
unpublished data), distributed from the coast to approximately 350m bottom depth. The
species is reported to occur both benthic and pelagic, and are often referred to as the
pelagic goby (Crawford et al. 1985), although this name seems not to reflect the true
nature of a primarily demersal species. However it commonly exhibits diel vertical
migration to midwater, and some life stages may also be found close to the surface
(O'Toole 1978, Crawford et al. 1985). The pelagic goby seems to be well adapted to the
often hypoxic environment of the Namibian shelf. The goby play a key role in the
ecosystem, both as predator on small prey as they feed on copepods and krill, but also
since it is abundant in the diet of a variety of commercially interesting fish species, most
noticeably the hakes, (Merluccius capensis and M. paradoxus), but also horse mackerel
(Andronov 1985), and seals and seabirds (Mecenero et al., in prep.)
Mesopelagic fish are part of a group of organisms referred to as micronekton, which
generally range in size from 1 to 15 cm (Salvanes & Kristoffersen 2001), with few
exceeding 30 cm in length (Smith & Heemstra 1991). These fish have a worldwide
distribution, oceanic as well as pseudo oceanic (neritic zone), and encompass more than
thirty described fish families (Gjøsæter & Kawaguchi 1980; Nelson 1994). They inhabit
the mesopelagic zone, generally described as the water column between 200 and 1 000m
depth, and are found in one or more of several deep scattering layers during daytime.
Some lanternfish (Myctophidae) and light fish (Photichthyidae) species as well as other
mesopelagic fish species occupying this zone migrate to shallower waters for feeding
purposes during night time, while others undertake only partial or no migrations at all
(Gjøsæter & Kawaguchi 1980; Prosch et al. 1995; Smith & Heemstra 1991). The diel
vertical migratory behaviour makes these fish vital transporters of organic matter
5
BCLME project LMR/CF/03/08
(nutrients) from the productive epipelagic zone down to the lesser productive aphotic
deep ocean. In other words "they form a vital link between the zooplankton community
and larger marine predators" (Prosch et al. 1995).
In the Benguela region many species belonging to most of the 30 odd families (Table 1)
have been observed and/or recorded in the catch logs of both research and commercial
fishing vessels. As with the goby, mesopelagic fish play a central part in the trophic
dynamics and ecology of the Benguela current system, preying mostly on zooplankton
and being preyed upon by fish, sharks, birds, and seals amongst others. Only one
lanternfish species has been of commercial interest the last three decades, being targeted
by the South African purse seine fishery. The question remains whether mesopelagic fish
species are a potentially harvestable resource, and if so, whether this can be done without
adverse effects on the entire Benguela system. Cruickshank (1982) argued that the full
role of these fish in the food web of the oceans was not known and that extensive
harvesting of these species should be monitored in order to avoid any detrimental effect
on the stocks of the predator species dependent on them.
6
BCLME project LMR/CF/03/08
2 Materials and Methods
The main objectives of this report are really twofold: a) to provide an overview of the
knowledge on the pelagic goby and mesopelagic fish species (mesopelagics) occurring in
the Benguela current system, and b) to present meta-data summary tables of data
collected onboard various research and fishing vessels that operated in the region. The
first objective included obtaining general and scientific information published in books,
scientific articles, thesis, and institutional reports, as well as popular articles. Regarding
the Benguela region the bulk of research done on mesopelagics has been focused on
members of the lanternfish (Myctophidae) and the hatchet fish Maurolicus muelleri
(Sternoptychidae). There is an apparent lack of knowledge and information on
mesopelagics occurring north of 15°S, as well as families other than the Myctophidae and
Sternoptychidae. The information available on southern and northern Benguela
mesopelagics was in many cases supplemented with knowledge on the same or similar
families/species studied in other marine systems. This information is generally mentioned
separately in and at the beginning of each sub-section. For the second objective metadata
tables were compiled for the Nan-Sis database (located at the Institute of Marine
Research, Bergen), as well as data available from various research and fishing vessels
that have worked or still are operating in Namibian (data located at NatMIRC,
Swakopmund) and South African (data located at MCM, Cape Town) waters.
Additionally a number of people working at various research institutions and also fishing
companies or associations were contacted in order to obtain additional inputs (Annex 16).
2.1 Regional data sources
Typically gobies and mesopelagic species have not been target species during regular
fisheries surveys within the region. However during cruises with these vessels incidental
catches of gobies and mesopelagic fish have been recorded.
Only two databases have been used more extensively in this study. These are described in
more detail below.
2.1.1 Nan-Sis
The Nansen Survey Information System (Nan-Sis) is currently an MS-DOS based
database programme designed primarily to capture and store station, catch and biological
data collected during scientific cruises onboard the Norwegian research vessel RV Dr.
Fridtjof Nansen (Strømme 1992). In recent years the Namibian Ministry of Fisheries and
Marine Resources (MFMR) has also used this database to capture bottom trawl survey
data collected onboard commercial fishing boats.
Strømme (1992) gives a detailed description and outline of the database. The database is
made up of several region or country specific projects (Table 3 in results section), and
species catalogues relevant to those regions or countries. In essence three types of data
are collected: a) station data, b) catch data, and c) biological data. Station data includes
information relevant to a uniquely numbered sampling station. The information captured
is the date, positional coordinates, type of gear used, sampling and bottom depth, duration
of a tow, i.e. tow start and end, speed, and tow distance. Each station number has unique
7
BCLME project LMR/CF/03/08
catch data linked to it. The catch data informs on type, quantity and weight of species
identified in a sample. Species common in an area are stored in a species catalogue,
where the full Latin and common name is linked to a species-specific identifier, i.e.
species code. It is this species code that is stored in the databases interface when
information is entered. Should biological data be collected for a certain species, for
instance in the form of length frequencies, sex or weight, a biological sample number is
linked to that species code entered under a unique station number in the catch data
interface.
For the purpose of this report we extracted station and catch data linked to 18 selected
fish families that include mesopelagic species (shown in bold in Table 1). Each project
linked to the Benguela region and stored in the database was searched for every species
code separately. These species codes are shown in Appendix 4 7.
MCM data
The RV Africana is one of four research vessels of the Cape Town based research
institute Marine & and Coastal Management (MCM). It has been used since the mid
1980's to conduct pelagic and demersal surveys along the southern African coast. During
these surveys, besides the usual station and catch information, various different fish
species were identified and data on them recorded. The data is stored in the `Africana
Demersal Data System' and each survey saved as a separate data file. This makes it
somewhat cumbersome to extract data, and programming is required to find the desired
data. Although species codes exist for most of the species, fish were generally only
classified to the family or genus level. A summary of these species and genus codes is
provided in Appendix 8 together with the number of stations for every species or genus.
Since the Africana surveys usually do not extend beyond the 450 m isobath, and many of
the relevant species occur beyond this depth, the data quantity for some of the genera and
species is very limited. In addition, the Africana trawl gear does not include a fine mesh
cod-end liner, resulting in many small fish escaping through the large meshes and thus
even less mesopelagic specimens caught.
The data from the Africana used in this report was kindly provided by Tracey
Fairweather from MCM, Cape Town.
2.1.2 NatMIRC data
The bulk of the Namibian research data was collected onboard the RV Welwitchia and
the RV Benguela I and II. Data on gobies and mesopelagics was generally not collected
systematically during Namibian research surveys and are located in the different research
programs at NatMIRC, making it difficult to obtain the data. Table 9 lists various data
sources and their possible locations, while some data from recent horse mackerel surveys
with the RV Welwitchia received for this report showing catch locations of gobies and
mesopelagics is presented in Annex 13.
The overview of the Namibian data used in this report was kindly provided by Angie
Kanandjembo from NatMIRC, Swakopmund
8
BCLME project LMR/CF/03/08
3 Overview and Results
3.1 Gobies
3.1.1 Species identity and diversity
According to Fishbase (Froese & Pauly 2005) the family Gobiidae is the largest family of
marine fishes in the world with possibly > 2,000 species. Also according to the same
source several species have been reported from South African (25), Namibian (6) and
Angolan (8) territorial waters. A closer look at the registered data leads one to conclude
that some of the reported species in this region must have been misidentified or
alternatively that the distributional range has been wrongly reported, and that the actual
number of marine goby species in the region is lower. However this report will not
address this as it only deals with the Sufflogobius bibarbatus (von Bonde 1923). The
species is popularly called the pelagic goby or also bearded goby. The name `pelagic'
may be misleading since this species, as most other goby species, is primarily demersal
but may as other demersal fish species exhibit diel vertical migration behaviour. S.
bibarbatus is endemic to the Benguela ecosystem and it is abundant throughout the
northern Benguela where it plays a key role within this ecosystem (O'Toole 1978;
Shannon & Jarre-Teigman 1999). The first record in Namibia has commonly been
reported to be the study by Barber and Haedrich (1969) while the species has been known
much longer in South Africa (von Bonde 1923). However a scientific reports from the
1920's found in the NatMIRC library show that the pelagic goby was reported off central
Namibia at that time (von Bonde 1928; Bronwen Currie, NatMIRC, pers. com.). The
main historic source for information on the gobies in the Benguela ecosystem are
publications derived from the extensive monitoring program off Namibia during the
1970's, namely the South West Africa Pelagic Egg and Larvae Surveys (SWAPELS). Of
all the publications from these surveys we wish to draw attention to the works by
O'Toole (1976, 1977 and 1978) who offers a comprehensive overview of the early life
stages of the goby and its distribution, which this report only give an overview of.
3.1.2 Species distribution
The distributional range of the pelagic goby has been reported by several authors mainly
during the late 1970's and the beginning of the 1980's. O'Toole (1976) reporting from
the SWAPEL surveys in 1972-73 found gobies to be abundant and widely distributed
between Hollams Bird Island (24°38'S) and Möwe Point (19°23'S) and up to 85 km
offshore. The southern limit was not defined because the survey did not extend south of
Hollams Bird Island. The recorded range in South Africa was defined from St. Helena
Bay on the west coast to St. Sebastian Bay on the Southeast coast. Cruickshank et al.
(1980) reviewing the SWAPELS data from October 1978 to June 1979 extended the
distribution of gobies in South African waters northwards across the border to Namiba to
roughly 28°00'S. The distribution in northern Namibia was extended from Möwe Point to
north of Cape Frio (17°40'S), and in the south from Hollams Bird Island (24°38'S) to
Lüderitz (26°30'S). (Refer also to O'Toole (1978), Cruickshank (1980), and Le Clus et
al. (2002)). This extension of the distribution is attributed mainly to increased data
sampling, but the authors still reported a gap in the distribution between 26°30'S and
28°00'S. New data collected with the RV Dr. Fridtjof Nansen in the region between 1990
and 2005 extend the distribution reported by the previous authors (Smith 1965; O'Toole
9
BCLME project LMR/CF/03/08
1976 & 1978; Cruikshank et al. 1980) further and show that the distribution is continuous
between South African and Namibian waters (Figure 2 and 3; Nansen programme
unpublished data). The data available show that the S. bibarbatus has been found
throughout the region from Tiger Bay at 16°50'S in Angola, along the shelf of Namibia
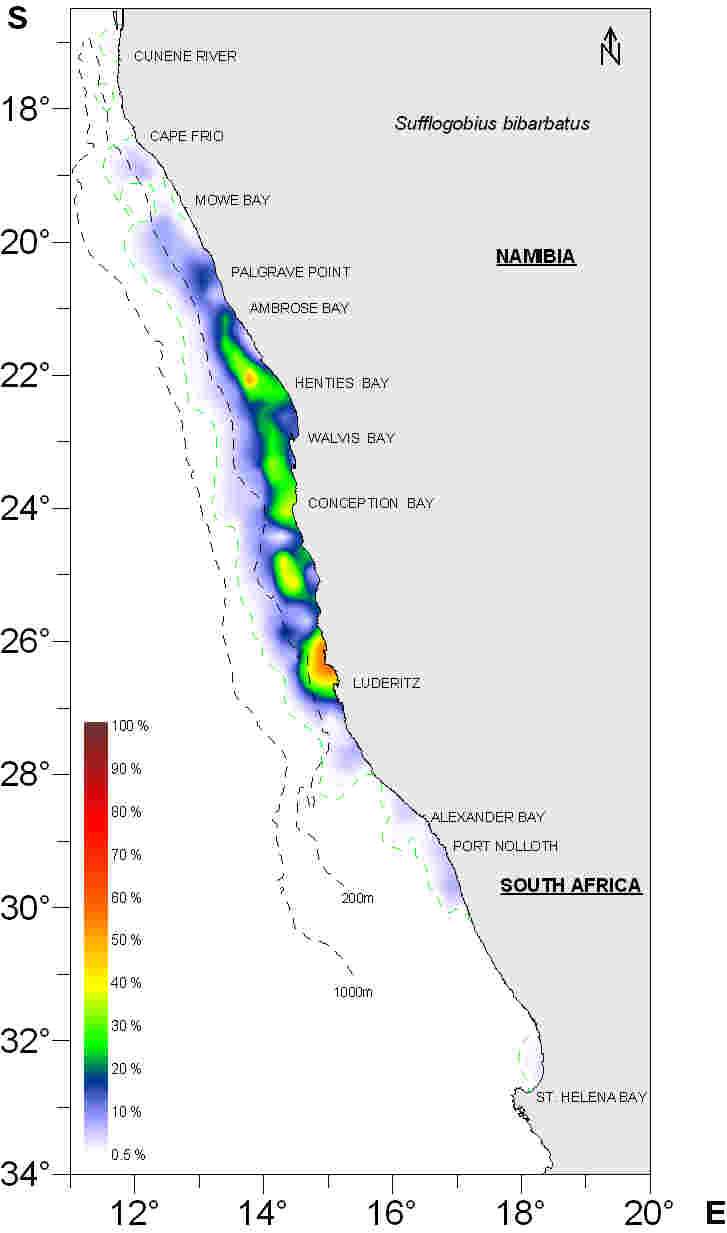
to south of 34°00'S in South Africa. The main concentration can be found in the central
part of this area from Ambrose Bay to north of Lüderitz in Namibia.
The distribution of S. bibarbatus presented in Figure 2 and Figure 3 has been calculated
using data from Nan-Sis and the Africana Demersal Data System from the period 1990
2005. All trawl catches during the time period have been divided into 20 x 10 nm
squares, and the frequency of occurrence calculated and plotted to illustrate the
distribution of S. bibarbatus in two different ways. Figure 2 takes only presence or
absence of gobies in trawl catches into consideration, and gives an overview of the total
area of distribution of gobies within the region, represented by the green line. This area
mainly corresponds with the shelf area, but with a more narrow distribution along the
coast south of Alexander Bay. Figure 3 takes into consideration the relative proportion of
gobies in each trawl catch and gives and overview of where the occurrence of gobies in
the catches is highest relative to other species. It is evident from Figure 3 that the highest
abundance of gobies in the catches is found on the central Namibian shelf (also see
Figure 4). It is noteworthy to mention here that the main abundance of gobies
corresponds with the diatomaceous mud belt characterised by regular periodic high
sulphur concentrations and anoxic water.
O'Toole (1976) suggested that there might be two populations of the pelagic goby in the
northern Benguela, based on observations of two different core spawning areas with
different peak spawning periods 200-400 nm apart. It was considered unlikely though that
the small poor-swimming goby would be able migrate these distances. This hypothesis
has recently been strengthened by a genetic study by De Silva (2005) based on the PCR-
RFLP analysis of the mitochondrial control region and the ND3/4 region. The study
shows that the goby populations from Lüderitz in the south differ genetically from those
off Walvis Bay (central) and Ambrose Bay (north). It is assumed that the difference is
due to the complex circulation pattern and existing oceanographic barriers around
Lüderitz.
10
BCLME project LMR/CF/03/08
S
0 0 0
0 0 0.1
CUNENE RIVER
0 0 0.02 0
18° 0.060.060
0 0.090.1 0
CAPE FRIO
Sufflogobius bibarbatus
0.1 0.2 0.2 0
0 0.2 0.4 0.5 0.2
MOWE BAY
20°
NAMIBIA
PALGRAVE POINT
AMBROSE BAY
0 0.030.3 0.7 0.80.09
0 0 0.070.7 0.6 0.6 0.2
0 0.020.6 0.7 0.4 0.40.06
0 0.2 0.7 0.6 0.4 0.20.04
0 0 0 0.7 0.6 0.30.060.05
0 0 0.040.6 0.6 0.4 0.2
0 0 0.1 0.6 0.5 0.5 0
22°
0.7 0.5 0.5 0
0 0.2 0.7 0.7 0.5 0.30.08
0 0 0.5 0.3 0.6 0.3
HENTIES BAY
0.9 0.8 0.5 0.7 0.3
0.06 0 0.4 0.8
0.4 0.4 0.5
0.020.020.4 0.9 0.6 0.5 0.5 0.3 0.3
0 0.050.7
WALVIS BAY
0.6 0.7
0 0 0.060.6 0.6 0.5
0.8 0.7 0.5
0.7 0.3
0 0.090.6
0.5
24°
0.060.8 0.9 0.8 0.5 0.7
0
CONCEPTION BAY
0.8 0.6 0.6
0 0.1 0.8 0.9 0.9 0.8 0.7
0.5 0 0.1
0 0 0.050.9
0.020.6 0.9
0.5 0.5 0.3
0
0.1 0.9 0.7 0.6 1 0.3 0.1
0 0 0.5 0.8 0.5
1 0.6
0
26°
0 1 1 0.7
0
0 0
1
0 0 0.3 0.8 0.8 0.9
1
0 0 0 0.1 0.7 0.9 0.8
0.1 0.3 0.8 0.9
LUDERITZ
0.1 0.9 0.8 0.9
0 0 0
0 0
0 0.4 0.8 0.7
1
0 0
0.040.2 0.7 0.8
0
28°
0.8
0.080.3 0.9
0.9
0
0.4 0
0.3 0.7 0.6
0 0.4
0 0.050.1
0 0.2 0.6
0.3 0.7
0.8
0.8
ALEXANDER BAY
0 0 0.1
0.5
0
0.1 0.1
0.6 0.9
0.4 0.4
0 0 0.05
0
0 0.1
0.9
PORT NOLLOTH
0
0.4 0.3
0.7
0
0 0 0
0 0
0.8
0 0
0
0.7
0.060.4
30°
0 0 0
0
0.8
SOUTH AFRICA
0 0
0 0
0.2
0
0.6
0
200m
0 0 0
0
0.5
0 0
0 0
0
0
0
0 0 0
0
0
0.3
0 0
0
0.1
0
0.5
0
0 0 0
0
0 0
0.2
0
0
0
0
0
0 0
0
0.4
0 0
0.1
0
0.4
0 0
0
0
0
0.3
1000m
0
0
0
0
0
0.7
0 0
0
0.2
0
0.2
32°
0
0.08
0.3
0
0
0.9
0
0.5
0
0
0.08
0
0
0
0
0.7
0
0
0.7
0
0
0.1
0
0
0
0
0.2
0
0.4
0
0.9
0
0
0
0.06
0
0
0
ST. HELENA BAY
0
0
0.1
0
0
0
0
0.1
0
0
0
0
0
34°
0
0
0.03
0
0
12°
14°
16°
18°
20°
E
Figure 2: Distribution of Sufflogobius bibarbatus in the Benguela. The figure represents a measure of
frequency of occurrence from all trawl hauls captured in Nan-Sis and the Africana Demersal Data System.
The data was collected on surveys conducted with RV Dr. Fridtjof Nansen between 1990-2005, Namibian
commercial vessels used for the hake swept area trawl surveys in the period 2000-2005 and the RV
Africana in the period 1985-2004. The green line outlines the maximum distribution area (refer to text for
details).
11
BCLME project LMR/CF/03/08
Figure 3: Distribution of Sufflogobius bibarbatus in the Benguela. The figure represents a measure of mean
relative abundance of gobies from all trawl hauls captured in Nan-Sis and the Africana Demersal Data
System. The data was collected on surveys conducted with RV Dr. Fridtjof Nansen between 1990-2005,
Namibian commercial vessels used for the hake swept area trawl surveys in the period 2000-2005 and the
RV Africana in the period 1985-2004.
12
BCLME project LMR/CF/03/08
% of hauls with gobies
Mean catch rate of gobies kg/hr
50,0
10,0
9,0
40,0
8,0
7,0
30,0
6,0
5,0
o
f
t
o
t
a
l
20,0
%
4,0
3,0
10,0
2,0
e
a
n
c
a
t
c
h
r
a
t
e
k
g
/
h
r
M
1,0
0,0
0,0
-37 -35 -33 -31 -29 -27 -25 -23 -21 -19 -17 -15 -11
Deg. Latitude south
Figure 4: Frequency of trawl hauls and mean catch rate of gobies per latitude - data from all swept area
trawl hauls made by the RV Dr. Fridtjof Nansen in the period 1990 to 2005, Namibian commercial vessels
used for the hake swept area trawl surveys in the period 2000-2005 and the RV Africana in the period
1985-2004 was combined.
Seasonal
Little information is available on changes in seasonal distribution patterns. However,
O'Toole (1976) reported that larvae and early juvenile stages were more widespread in
summer than during the winter both offshore and along the coast. O'Toole (1977) did not
find any seasonal differences for adult fish. Mecenero (2005) reported that the frequency
of gobies in the diet of seals at Cape Cross was highest during the period from August to
January, possibly an indication that other food items were less available during that
period. At Atlas Wolf Bay and Van Reenen Bay the frequency of gobies in the seal diet
was highest in the period February to July, the same period as the upwelling is at a
minimum. A clear seasonal difference in fish size was observed, with much larger fish in
the seal diet in the period August to January than during January to August. The observed
differences reported in this study could either be attributed to real differences in goby
behaviour or seasonal differences in the seals foraging pattern.
Distribution Inshore-Offshore
In general all observations indicate that larger gobies are found further offshore and are
considered more demersal than smaller specimen (O'Toole 1978; Nansen programme,
Unpublished data). According to Le Clus et al. (2002) based on data from the SWAPEL
surveys in 1978 and 1979, the cross-shelf abundance of young males and females in the
pelagic zone peaked 10-15nm offshore and petered out about 35nm from the coast. This
distribution is consistent with a two-celled cross-shelf circulation model (Barange &
Pillar 1992) whereby pelagic goby, phytoplankton and the euphausiid Nyctiphanes
capensis are concentrated inshore of the coastal upwelling front. Young gobies occured
13
BCLME project LMR/CF/03/08
further offshore during November-April, a period of low upwelling, compared to August-
October when upwelling is usually more pronounced. The along-shore abundance of
young gobies peaked between 2122°S and between 2326°S, concomitant with local
peaks in chlorophyll a. Analyses of catch data from the Nan-Sis database showed that the
highest catch rates were between 100200m depth and that the gobies are frequently
found at a depth of 300m bottom depth and occasionally to a max depth of 350 m (Figure
5). No catches were reported or registered offshore of 400m depth. It is difficult to
establish any distributional limit in the shallow area due to the low number of trawl
stations inshore of 50m bottom depth, but gobies are frequently found inshore to 50m
depth, with decreasing catch rates inshore of 100 m depth (Figure 5). The distribution
becomes shallower in the low-density areas south of Lüderitz (Figure 3).
Average catch/nm2
Number of st. with gobies
Total number of stations
25
200
180
20
160
2
140
15
120
100
a
t
c
h
t
/
n
m
10
80
C
b
e
r
o
f
s
t
a
t
i
o
n
s
60
u
m
N
5
40
20
0
0
0
50
100
150
200
250
300
350
400
Depth (m)
Figure 5: Catches of gobies per depth stratum off Namibia. Catch rates in t/nm2, number of stations with
catches of gobies and total number of trawl stations.
Diurnal
The bearded goby S. bibarbatus is a major component of the sound scattering layer over
the continental shelf in the northern Benguela region, and its diurnal behaviour has been
recorded hydro-acoustically (Le Clus et al. 2002; Nansen programme, unpublished data;
Salvanes et al. 2004). Generally the goby stays closer to the bottom during the day and
higher in the water column at night, but variations in this general pattern have been
observed. Larger gobies have been reported to exhibit less diurnal migration tendency
than smaller ones, and observations where gobies stayed close to the bottom or higher in
the water column with no apparent diurnal migration have been made (Salvanes et al.
2004; Rengqe 2005; Krakstad et al. 2006). Catches of gobies support the general finding
that gobies stay in the pelagic zone during the night and near the sea floor during the day
14
BCLME project LMR/CF/03/08
(Le Clus et al. 2002; Salvanes et al. 2004; Krakstad et al. 2006; Nansen programme,
unpublished data).
Environmental parameters
The goby is an opportunistic species living in the inner shelf environment of Namibia
where large fluctuations in environmental parameters, particularly oxygen, sulphur and
temperature, can be observed regularly (Hamukuaya et al. 2001). Hamukuaya et al.
(2001) reported that this inner shelf environment was typically characterised by bottom
temperatures between 10-12.5°C, a salinity of 34.8-35.2ppt , and dissolved oxygen <1
ml/l, with all values observed for average bottom depth <200 m. However these are
bottom average measurements only and the major part of the goby resource is found in
pelagic water masses. O'Toole (1976) reported main concentrations of adult gobies in
water masses with temperatures from 14.5°C to 17,5°C, and juveniles in water masses
from11-15°C. More recently several surveys focusing on the behaviour and tolerance of
gobies to environmental parameters have been undertaken. During a survey in 2003 off
Namibia, gobies tended to be associated with depths of low oxygen concentrations.
Trawls often gave high catches in oxygen concentrations of 0.2-1.0 ml/l (Salvanes et al.
2004). Adults tended to be present at lower oxygen concentrations as well as deeper than
juveniles.
Shipboard experiments conducted during 2003 off Namibia indicated that gobies are
exceptionally tolerant to anoxic and sulphuric water. Recovery following exposure to
total anoxia and concentrations of up to 100umol·l-1 of sulphide indicated some
specialized physiological adaptation - there were no indications in the behaviour of the
goby of an escape strategy (Salvanes et al. 2004). During the experiments onboard Dr.
Fridtjof Nansen in 2006, adult gobies showed a remarkably high tolerance to low
concentrations of dissolved oxygen. Their response to decreasing oxygen levels was an
intermediate increase in the gill ventilation volume and frequency at ca 0.2ml DO/l
(called critical oxygen level) followed by a sudden drop in the gill beat frequency
(<0.2mlDO/l). The critical oxygen level is the oxygen level at which fish shift from
aerobic to anaerobic metabolism. The gobies tolerated 4.5 hours at oxygen levels of <
0.01ml DO/l without showing signs of equilibrium loss. When oxygen levels increased
after having been under oxygen stress for up to 9 hours the gobies recovered rapidly to
normal breathing and behaviour. When the fish were "attacked" (poked by a stick) after
having been in < 0.01ml DO/l for 4.5 hours, they responded immediately with an escape
response, implying that their brain was `turned on' also after a long time of anaerobic
metabolism (Krakstad et al. 2006). These observations probably illustrate rather typical
values. It has been suggested that gobies may be using oxygen from their inflated swim
bladder (O'Toole 1976) while they are passively hiding from predators in oxygen poor
layers during the day, and that they migrate to the surface at night partly to refill this
supply of oxygen.
3.1.3 Biology
The goby has generally been recorded on soft bottom communities. Goosen et al. (2000)
analysed videotapes taken from the research submersible Jago operating off the Orange
River Mouth in 1996 and 1997. They found that nekton communities on soft sediments
15
BCLME project LMR/CF/03/08
were dominated by S. bibarbatus, together with juvenile hake, cuttlefish, false jacopever,
and kingklip. It has been suggested that the distribution roughly overlays the
diatomaceous mud belt off Namibia, and more recently that this also coincided with the
main distribution area for gelatinous zooplankton (Jellyfish) in the region (Krakstad et al.
2006).
Observations of the goby in situ and in aquaria showed that it is a poor swimmer and may
remain relatively inactive over long periods (O'Toole 1976; Goosen et al. 2000; Salvanes
et al. 2004; Krakstad et al. 2006). The colouration and pigmentation has been described
by O'Toole (1976), and the species is able to change coloration (darker-lighter) according
to the substrate (pers com A.C. Palm, University of Bergen).
3.1.4 Ecology
According to Crawford et al. (1987) Barber and Haedrich (1969) found phytoplankton of
the genus Delphineis (=Fragilaria) karstenii and Coscinodiscus spp. to dominate the diet
of juvenile gobies, while Ryther (1969, as referred to by Crawford et al. 1987) also drew
attention to the importance of the large, chain forming Delphineis in the food of gobies.
O'Toole (1978) found that the diet of adults, juveniles, and larvae consisted
predominantly of Delphineis and Chaetoceros, although he also observed remains of
copepods and euphausiids. However D'Arcangues (1976) in her study found mainly
copepods and euphausiids in the stomachs of the juvenile and adult gobies. Crawford et
al. (1987) found that in 1979-1981 gobies contained numerically 93% phytoplankton
(mostly diatoms) and 7% zooplankton. Zooplankton was present in 80% of the stomachs
examined and phytoplankton in 90%. Delphineis karslenii was the most abundant
phytoplankton species, with Chaetoceros and Coscinodiscus spp. also eaten, and
euphausiids and copepods were the dominant zooplankton groups (Crawford et al. 1985).
Similarity between the diet of gobies and of adult pilchard was noted by Crawford el al.
(1985), who considered it likely that in the intense perennial upwelling system situated
between 22°S and 27°S gobies partially replaced pilchards during the 1970s. A new study
on the diet of gobies in the northern Bengula, also comparing between depth and the type
of prey found in stomachs, is underway. The gobies collected for this study were mainly
collected with a bottom trawl. Preliminary results show that small crustaceans (mostly
euphausiids) dominate the diet at depths less than 200 m, while polychaetes dominate the
stomach content of fish caught at greater depths. This study also indicates that gobies
feed throughout the day, with a probable peak in feeding at early morning and early
evening. Although fish of all sizes fed on similar prey, larger fish appeared to feed on
larger quantities and larger individuals of prey (Vaarland et al. 2002; M. Gibbons,
University of Western Cape, pers. com.). Preliminary result from the goby surveys in
2004 and 2006 (Salvanes et al. 2004; Krakstad et al. 2006) showed the presence of
crustaceans, mainly copepods and amphipods, polychaeta, fish larvae, including own
larvae and bottom sediment - mainly dead diatoms and sulphur bacteria. The information
available suggests that S. bibarbatus is an opportunistic feeder that takes what it is
presented with. Sulphur bacteria have not before been registered in goby stomachs.
16
BCLME project LMR/CF/03/08
Crawford et al. (1987) give a summary of the major predators of the bearded goby.
During the 1970s and early 1980s pelagic gobies were a major food item for many
species off Namibia, including cape horse mackerel (Trachurus trachurus capensis;
Venter 1976), cape hakes (Merluccius capensis; Chlapowski 1977; Assorov & Kalimna
1979; Prenski 1980; Andronov 1983; Konchina 1986), kingklip (Genypterus capensis;
Macpherson 1983), monk (Lophius vomerinus; Macpherson 1985), large-eye dentex
(Dentex macrophthalmus; Kuderskaya 1985), west coast sole (Austroglossus microlepis;
A. Badenhorst, pers. com.), some coastal-breeding seabirds, and the cape fur seal
(Arctocephalus pusillus pusillus; Crawford et al. 1985). Not exploited commercially to
any great extent, the pelagic goby must therefore be of considerable importance in the
northern Benguela ecosystem.
3.1.5 Growth parameters
A combined length-weight relationship for both goby sexes has been published by a)
Cruickshank et al. (1980), while Melo and Le Clus (2005) published age-weight
relationships for each sex separately, b) observing that the growth rate in males is faster
than females. More details on the measurements can be found in the respective
publications.
a)
W = 0.0143L 3.0543
c
R2 = 0.97
N = 200
b1)
Males
W = 1.105e0.4838x
R2 = 0.7427 N = 72, excl. back calc.
b2)
Females
W = 1.363e0.3448x
R2 = 0.5194 N = 40, excl. back calc.
Where:
W = Weight measured to the nearest 0.1 grams
Lc = Caudal length measured to the nearest millimetre below
x = Number of hyaline zones
S. bibarbatus can attain a length of 13 cm (Hoese 1991) and an age of 6 years (Melo &
Le Clus 2005). Mecenero et al. (in press) report on remnants of gobies with 17 cm in
length, based on back calculated length estimates from otholits found in seal scats caught
at the Atlas-Wolf Bay south of Lüderitz. Unpublished data collected during hake
abundance surveys off Namibia with the RV Dr. Fridtjof Nansen also shows gobies of
this size, with two records in the database showing gobies with a total length of 20 and
21cm.
3.1.6 Reproduction
The main spawning season for pelagic goby has been reported to be from July to
February, with a peak in late winter to early spring (O'Toole 1977). Spawning has been
reported to be most intense in costal waters south of Walvis Bay, but with less intense
spawning over a more extended area during summer (O'Toole 1977).
The reproduction of S. bibarbatus as reported by Melo and Le Clus (2005) suggests a late
maturation at 2-3 years of age, and males maturing at a greater size and age than females.
They observed that two batches of yolked oocytes were present in the ovaries during their
17
BCLME project LMR/CF/03/08
study and that the maximum gonadosomatic index was 14.3%. Batch fecundity was
significantly correlated with standard length, R2 = 0.88, and ovary-free body weight, R2=
0.92. Fecundity ranged from about 2 000 eggs in females 5.0-5.5cm long to about 10 000
eggs in a female 9.8cm long. The mean fecundity was 842 ±189 eggs per gram of ovary-
free body weight. Melo and Le Clus (2005) suggest that the pelagic goby may be a serial
batch spawner, based on the extended spawning season from July to April, and the
presence of more than one batch of yolked oocytes in the ovaries.
The SWAPEL surveys in the 1970's investigated the presence of egg and larvae in the
upper part of the water column <50 m depth. While goby larvae were common in pelagic
plankton net hauls and pelagic trawl hauls goby eggs were never reported (O'Toole
1977). It was therefore suggested that the pelagic goby has demersal eggs like several
other goby species around the world. This however has still not been verified.
The larval morphology, pigmentation and development has been described in details and
the reader is referred to O'Toole (1976, 1977) for a comprehensive overview.
3.1.7 Abundance
Little information on biomass is available from the literature on the abundance of S.
bibarbatus. Circumstantial evidence from diet studies of the cape gannet (Morus
capensis), jackass penguin (Spheniscus demersus) and the cape comorant (Phalacrocorax
capensis) during 1957-58 and in 1980 suggests that the goby resource was relatively low
in the period of the first study (Crawford et al. 1985), since gobies were not present in the
diet of these bird species in 1957-58, but frequent in 1980. Also, according to Crawford
et al. (1985) gobies were not registered in Purse seine catches before 1972. Observations
from plankton surveys in the 1960's off Namibia did not register gobies while the species
had become abundant during the SWAPEL surveys in 1972-1973 and 1978-1979
(O'Toole 1976, 1978). However, reports from scientific surveys from the 1920's found in
the NatMIRC library show that the goby was found off central Namibia in the same areas
as they occur in these days. No quantitative information was given but the number of
stations where gobies were present indicates that it was common in that period (B. Currie,
NatMIRC, pers. com). Generally two published biomass estimates exists. Hewitson and
Cruickshank (1993) estimated a biomass of 600 000mt based on data from bongo net
hauls during the SWAPEL surveys in 1978-79. The initial biomass of the surveyed fish
was calculated to be 150 000mt (based on data from the upper 50 m), but the total
biomass was assumed to be four times larger. A raising factor of 4 based on several
considerations was summarized to the effect that the rest of the water column and the
bottom layer contained higher densities of fish than the surface layer. A further estimate
of abundance was presented by Shannon and Jarre-Teichmann (1999). Based on the
results of an ECOPATH model they present an estimated average biomass of 1.45 106mt
for the northern Benguela during the 1980's.
Recent estimates of demersal goby abundace based on day time trawl data from swept
area bottom trawl surveys conducted with the Dr. Fridtjof Nansen suggest that the
demersal component could have been in the range of 10 000 - 100 000mt during the
1990's, increasing in the last part of the period until 2005 (Figure 6; Nansen Programme,
18
BCLME project LMR/CF/03/08
unpublished data). Note that the demersal surveys did not cover the full distributional
range of the gobys and also that no estimate of the pelagic component was made. The
estimates are therefore lower than the actual abundance and should be treated as an index
only. The pelagic component is possibly the larger part of the resource, although two
dedicated goby surveys in 2004 and 2006 (Salvanes et al. 2004; Krakstad et al. 2006)
focusing on the goby's diel vertical migration indicated that the pelagic component may
be smaller than previously assumed. However, applying similar assumptions to the
Hewitson and Cruickshank (1993) estimate, the pelagic component may be between 3
and 5 times that of the demersal component. From these results it seems that the estimate
from the ECOPATH results for the 1980's is unrealistically high and does not represent
the situation during the 1990's. Also, during the 1990's the pelagic purse seine industry
in Namibia has been in an almost constant crisis with decreasing catches of all clupeid
species. Despite this and the fact that the pelagic goby is slow moving and distributed
over a well-defined area, only sporadic catches of this species occurred in the landing
statistics, suggesting that the species might be less abundant than often believed.
100000
80000
60000
a
s
s
(
t
o
n
n
e
s
)
40000
i
o
m
B
20000
0
jan.90jan.91jan.92jan.93jan.94jan.95jan.96jan.97jan.98jan.99jan.00jan.01jan.02jan.03jan.04jan.05
Month / Year
Figure 6: Abundance of Sufflogobius bibarbatus calculated from the swept area trawl surveys conducted in
Namibia from 1990 until present. The figure only represents the biomass of gobies that stayed on the
bottom during the survey period (during day time). Also the surveys did not cover the full distributional
range of the goby resource. Therefor these estimates should be treated as an index only.
19
BCLME project LMR/CF/03/08
3.1.8 Catch history
The S. bibarbatus has never been targeted commercially in Namibia or South Africa.
Bycatch of the species occurs both in the pelagic fishery, the midwater fishery and the
demersal fishery, but the mesh size regulation enforced on the midwater and demersal
fisheries probably prevent any large catches in the fishery being made. Also, infrequent
low catches of low value small sized fish are probably either dumped or made into
fishmeal, thus catches from these fisheries have only rarely been reported. The purse
seine fishery operates with a finer (sardine, or anchovy) net mesh, and irregular records
of gobies are registered in that fishery. Annex 14 gives yearly catches of gobies in
Namibia and South Africa as reported by the FAO (www.fao.org/fi). The catches vary
substantially from year to year but are generally very low. Average catches of 216
tons/year are reported in Shannon and Jarre-Teichmann (1999), but no information on
where this information comes from.
20
BCLME project LMR/CF/03/08
3.2 Mesopelagics
3.2.1 Species identity and diversity
Mesopelagic fish have a worldwide distribution, oceanic as well as pseudo oceanic, and
encompass more than thirty fish families (Gjøsæter & Kawaguchi 1980; Nelson 1994).
The taxonomic arrangement and naming of some of the families listed in Table 1 might
differ between various classification systems. For instance, in FishBase (Froese and Pauly
2005) the families Chauliodontidae, Astronesthidae, Idiacanthidae, Malacosteidae, and
Melanostomatidae are listed as subfamilies of the family Stomiidae.
Table 1: Families of mesopelagic fish with corresponding numbers of genera. Families shown in
bold were searched for in the Nan-Sis database.
Order
Family
Common name
FishBase
Gjøsæter &
Smith &
2005
Kawaguchi 1980 Heemstra 1991
Anguilliformes
Nemichthyidae
snipe eels
3
5
2
Argentiformes
Argentinidae
argentines
2
2
4
Ophistoproctidae
barreleyes
6
4
3
Bathylagidae
deep-sea smelts
1
2
1
Platytroctidae
tubeshoulders
13
6
Stomiiformes
Stomiidae
scaly dragonfish
27
2
2
Chauliodontidae
viperfishes
*
1
1
Astronesthidae
snaggletooths
*
6
3
Idiacanthidae
sawtail fishes
*
1
1
Malacosteidae
loosejaws
*
4
3
Phosichthyidae
lightfishes
7
7
Gonostomatidae
bristlemouths
7
20
6
Sternoptychidae
hatchetfishes
10
3
5
Melanostomatidae scaleless
dragonfish
*
15
10
Aulopiformes
Scopelarchidae
pearleyes
4
5
4
Giganturidae
telescopefish
2
2
1
Omosudidae
omosudids
1
1
1
Anotopteridae
daggertooths
1
1
1
Alepisauridae
lancetfishes
1
1
1
Paralepididae
barracudinas
12
5
7
Notosudidae
notosudids
3
3
Evermannellidae
sabretoothed
fishes
3
3
2
Myctophiformes Myctophidae
lanternfishes
32
30
28
Neoscopelidae
blackchins
3
2
Lampriformes
Trachypteridae
ribbonfishes
3
3
3
Lophotidae
crestfishes
2
2
2
Regalecidae
oarfishes
2
2
2
Beryciformes
Anoplogasteridae
flashlight fish
1
2
1
Melamphaidae
bigscale fish
5
2
5
Perciformes
Gobiidae
gobies
212
Chiasmodontidae
swallowers
4
5
3
Gempylidae
Snake mackerels
16
20
15
Trichiuridae
frostfishes
9
8
5
Centrolophidae
medusafishes
7
1
1
Tetragonuridae
squaretails
1
1
1
21
BCLME project LMR/CF/03/08
The most specious families are the Stomiidae, Myctophidae and Sternoptychidae (Nelson
1994), with 279, 247, and 71 species listed respectively (FishBase 2005). In terms of
genera per family the Gonostomatidae, Melanostomatidae, Myctophidae, and
Gempylidae are the most diverse (Gjøsæter & Kawaguchi 1980; Table 1). The numbers
of genera listed in Table 1 under Smith and Heemstra (1991) are those found in southern
African waters and differ to those listed under FishBase (2005) and Gjøsæter &
Kawaguchi (1980). For an overview of the various families and their corresponding
genera occurring in southern African waters, Smith and Heemstra (1991) provide detailed
descriptions of each family and its species, while additional information for some of these
species can be found in FishBase (2005). Picture plates of some selected mesopelagic
species are shown in Annex 1-3.
3.2.2 Species distribution
In the northern Benguela region off the Namibian coast Rubis (1985) reported on a total
of 41 myctophid species. Of these, twenty-five originated solely from the 400 mile
offshore Valdivia Bank on the Walvis Ridge, ten were specific to the northern Benguela
area, and six common in both areas (Rubies 1985). Two species belonging to the oceanic
lanternfish genus Symbolophorus and the pseudo oceanic warm-water genus Diaphus
were also common off the coast of Namibia (Prosch et al. 1995). Of the 14 species of
sternoptychids (hatchet fishes) found in South African waters, 11 have been recorded in
the eastern south Atlantic (Prosch et al. 1995).
Hulley (1991) gives a general account of 28 genera, comprising 125 species, of
myctophids likely to be found in the southern African region. This account covers the
detailed description by Hulley (1986) of the distribution of myctophids occurring in the
southern Benguela. This study describes 65 species in 23 genera in the area between
28040'S and 40000'S, and lists 61 oceanic myctophid species, describing their distribution
patterns according to Hulley (1981).
Geographic (horizontal)
The most frequently described lanternfish is Lampanyctodes hectoris, also the most
prominent pseudo oceanic myctophid species described for the Benguela region (Hulley
1986; Hulley and Lutjeharms 1989; Hulley 1992). Pseudo oceanic species generally
inhabit the pelagic and mesopelagic zone over the continental shelf and slope, and are
associated with land environments and land orientated food chains (Hulley 1986). In the
northern Benguela L. hectoris has been reported on the outer shelf edge more than 30
miles offshore as well as just 5 miles off the Lüderitz coast (Cruickshank 1982). O'Toole
(1976) also provided distribution maps of several myctophid species caught in bongo nets
in the northern Benguela between Hollams Bird Island and Cape Frio. According to his
findings the majority of L. hectoris were found 30 112km offshore, but also close
inshore (at 28m bottom depth) south of Walvis Bay. Other myctophids like
Symbolophorus boobs and several Diaphus species were found 30112km offshore
between 180S and 250S (O'Toole 1976).
In the southern Benguela data collected onboard the RV Africana in 1988 showed that S.
boobs occurred far offshore in waters deeper than 500m, while Diaphus hudsoni was
22
BCLME project LMR/CF/03/08
caught mainly deeper than 300m (Augustyn 1988). Hulley & Lutjeharms (1989), based
on catch data collected between 25030'S and 34055'S, grouped the lanternfish into two
groups, which were correlated to bottom depth. Inshore of the 800m isobath, L. hectoris
was the dominant species, while the `off-shore' group included the oceanic species C.
warmingii, D. hudsoni, D. meadi. A study on the genetic variation of the L. hectoris from
4 locations along the South African west coast showed little genetic differences,
suggesting a genetically homogenous population (Florence et al. 2002). In addition,
seasonal spawning populations of these fish seem to be confined to the continental slopes
(Prosch et al.1995).
Off southern Africa the occurrence of the hatchet fish Maurolicus muelleri is generally
confined to an area east of the thermal front, which is characterized by the upwelling of
water along the west coast (Prosch et al. 1995). They occur in Angolan waters and their
distribution extends all along the west coast to Cape Point in South Africa. Within this
area, their distribution is patchy with marked seasonal differences. A detailed account of
the distribution of M. muelleri in the southern Benguela is given by Armstrong & Prosch
(1991). This species, classified as a shelf resident, was recorded up to 100nm offshore,
with the highest densities observed between the 100 and 500m depth contours. Augustyn
and Hulley (1988) reported that few hatchetfish were caught beyond the 500 m isobath.
In the northern Benguela this species has been recorded in bongo net catches between 43
and 112km offshore, at bottom depths ranging from 140 to 670m (O'Toole, 1976).The
scaly dragon fish Stomias boa boa (Stomiidae) was found far offshore in the northern
Benguela. The maximum bottom depth of the stations where this species was sampled in
the upper 50m during night hours was 3000m (O'Toole, 1976).
Diurnal and vertical distribution
Mesopelagic fish perform diel vertical migrations to upper water layers mostly in search
of food. Some species only partially migrate during night hours (semi migrant species),
while some species are non-migrant (Watanabe 1999; Williams 2001). Even within a
species, this behaviour may vary depending on season, sex and age (Prosch et al. 1995).
Huse et al. (1998) described the diurnal vertical distribution of some lanternfish species
in the northern Benguela off Namibia. They observed four vertically migrating
mesopelagic layers at night, dominated by the myctophid species L. hectoris and S.
boobs. The hatchetfish M. muelleri was observed in all four mesopelagic layers and
progressively increased in size with increasing depth. Larger L. hectoris and S. boobs
were found in the top layer than in the second layer, but then increased in size with
increasing depth (Huse et al. 1998). Size stratification with depth is also exhibited by
some lanternfish in the southern Benguela (Hulley 1991).
Hulley & Prosch (1987) described L. hectoris' and M. muelleris' vertical distribution in
the southern Benguela, based on commercial catch data as well as research data. Highest
catch rates of L. hectoris were in the 101-200m and 201-300m depth ranges, while
highest catch rates in the 0-100m fishing depth were observed inside the 100m isobath,
suggesting an inshore (lateral vector) to the vertical migration (Hulley & Prosch 1987).
Based on data from 1988, Huley (1992) described the vertical distribution of 51
23
BCLME project LMR/CF/03/08
lanternfish fish species in the Cape Canyon and the Cape Point valley. He observed an
increase in species diversity with depth and simultaneously a decrease in catch rates. His
findings confirmed that L .hectoris was the dominant species at 300 m bottom depth, and
that the subantartic species dominated catches at deeper depths. Armstrong and Prosch
(1991) reported on the diel vertical migration behaviour of the lightfish M. muelleri. In
the southern Benguela this species ascends during the afternoon in dense schools. These
dense schools disperse into a diffuse scattering layer in the mid to upper water column
during darkness, and at dawn rise to the surface. Before sunrise these layers fish descend
in a narrow layer at a rate of 0.03m·s-1.
Seasonal
Information on seasonal influences on the distribution of mesopelagic fish is very limited,
and no dedicated studies on the effects of environmental factors on the distribution of
mesopelagic fish have been done. Many authors have shown though, that the oceanic
distribution of lanternfish in particular can be related to physical, chemical, and
biological characteristics of the water column (see references in Hulley 1992).
The little information that is available from the Benguela is not surprisingly on L.
hectoris. This species displayed a seasonal migratory behaviour, moving farther offshore
during low-level upwelling winter months in order to spawn (Hulley & Lutjeharms
1989). Mean catch rates during summer between 100 and 300m depth ranged from 4.9 to
5.6 specimens/hour, while in winter daily catch rates ranged from 73.6 to 153.9
specimens/hour (Hulley 1986). Additionally during summer months this species occurred
mainly inshore of the 300 m isobath, with an offshore limit at 500 m bottom depth. In
winter months this off shore limit extended to the 1000 m isobath. It is suggested that in
the southern Benguela, this distribution pattern is governed by frontal dynamics, which
influence the food availability during the different seasons. Such a seasonal distribution
pattern does not seem to apply to the oceanic myctophids (Hulley & Lutjeharms 1989).
Environmental parameters
Migrant mesopelagic species are described as eurythermal, occupying different
temperature ranges during the day and night habitat. Most non-migrants on the other hand
occupy a more temperature stable environment (Watanabe 1999). In the Northwest
Pacific mesopelagic species show a specific zoogeographical affinity, also associated
with hydrographic structures (Moku 2000).
Hulley (1992) found that in the southern Benguela, where the horizontal and vertical
temperature structuring of the water column was noticeable, the distribution of oceanic
lanternfish was limited by the 300 m isobath. He further pointed out that temperatures
and bottom depths correlated with the down slope distribution of fish species. Armstrong
and Prosch (1991) report that while performing diel vertical migrations, M. muelleri
experiences temperature differences of 100C, and that the horizontal distribution of M.
muelleri was not related to the temperature structure during two surveys done in the
southern Benguela in the 80's. Cruickshank (1982) reports that L. hectoris occurred in
waters with surface temperature of 100 to 250C, while Hulley and Lutjeharms (1989)
found only little correlation between relative abundance of L. hectoris and temperature,
24
BCLME project LMR/CF/03/08
with a tendency though of greater abundances found in waters with a sea-surface
temperature of less than 16,40C. Ahlstrom et al. (1976) reported that more than 60% of L.
hectoris larvae occurred where sea surface temperatures ranged between 14 0C and 15,5
0C .
3.2.3 Biology
Mesopelagic fish are generally small, mostly <30 cm. Many have large, sensitive eyes
and well-developed ventral and dorsal light organs that emit light in the visible spectrum
(Salvanes & Kristoffersen 2001; Smith & Heemstra 1991). Deep living fish have reduced
metabolic rates, low oxygen consumption and probably reduced swimming activity. On
the other hand migrating species have well developed muscles and gills, as well as large
hearts and usually swim bladders (Salvanes & Kristoffersen 2001). Some fish, like the L.
hectoris, build up lipid reserves during summer months, when food availability is high, to
use these reserves during the winter spawning months (Hulley & Lutjeharms 1989).
In the southern Benguela sex ratios of M. muelleri and L. hectoris were female biased
(Centurier-Harris 1974; Prosch 1991). Females migrating closer to the surface than males
during darkness as well as a sampling bias might explain the skewed ratio observed in
numbers of males and females observed (Hulley & Prosch 1987). Young et al. (1987)
also observed female biased sex ratios among L. hectoris off Tasmania, and suggested a
spatial segregation of the sexes as a possible reason. Other factors such as species size
and depth distribution can account for observed biased sex ratios (Young at al. 1987).
Crawford (1980) also reports on female biased sex ratios in commercial landings of L.
hectoris, and explains this with the fact that most catches were taken from January to
April, outside the main spawning season. This also suggests that there is some connection
between the sexual cycle and the pattern of distribution (Centurier-Harris 1974).
3.2.4 Ecology
As already mentioned many species migrate to the epipelagic zone at night in order to
feed on the abundant zooplankton of the surface waters, while others migrate only
partially or not at all (Hulley 1991; Smith & Heemstra 1991), feeding on zooplankton like
copepods, amphipods, euphausiids and fish (Oven 1990; Hopkins et al. 1996; Prosch et
al. 1995; Williams 2001; Watanabe 2002; Young & Blaber 1986). Feeding habits of the
four most common myctophid species found in the western North Pacific suggest
resource partitioning (Watanabe 2002). The fish species investigated migrated to the
upper 1m layer, and respectively fed mainly on euphausiids, amphipods,
appendicularians, and pteropods, which are all zooplankton species (Watanabe 2002).
Similarly, members of the lanternfish in the southwest Atlantic primarily feed on
copepods, amphipods, and euphausiids (Oven 1990). An investigation into the feeding
behaviour of myctophid and stomiiform fishes from the western North Pacific and off
southern Tasmania, showed non-migratory species feeding mainly on a single prey item,
while migratory species had a diel feeding pattern, feeding on different species during
night and day respectively (Moku 2000; Williams 2001). Also, while some species fed
throughout the diel cycle, others showed changes in the state of stomach fullness or so
called feeding periodicity, depending on the time of day. The feeding strategies of the
25
BCLME project LMR/CF/03/08
investigated species suggest a trade off between high and low energy demands of their
respective life styles (Moku 2000).
Hulley (1991) described the lanternfish as opportunistic feeders, preying on small
crustaceans, fish eggs, and fish larvae. In the southern Benguela L. hectoris preyed on a
range of crustaceans such as copepods (61.6%), amphipods (26.6%) and euphausiids
(11.6%) (Prosch et al. 1995; unpublished data in Prosch 1986). Hewitson and
Cruickshank (1993) estimated the consumption of meso - and macro zooplankton by
lanternfish in the northern Benguela at 1.65 106 tons. They used a 40:60 proportion of
meso- to macroplankton in the diet composition of lanternfish to estimate the total annual
consumption of zooplankton.
Mesopelagic fish are an important food source for fish such as the cape hake and deep-
sea hake (Assorov 1979; Huse 1998; Payne et al. 1987; Pillar & Barange 1997; Punt
1992; Traut 1996), horse mackerel (Andronov 1983; Konchina 1986), snoek (Nepgen
1979), orange roughy (Rosecchi 1988), various squaloid sharks (Ebert 1992),
cephalopods (Jackson, 1998; Villanueva 1993), seals (David 1987), various bird species
(Jackson 1988), and several cetaceans (Prosch et al. 1995).
Punt et al. (1992) estimated that in the southern Benguela, based on data from 1988 and
1990, hakes consumed an estimated 84 000 and 312 000 tons of myctophids annually.
When considering the estimated consumption of Stomiiformes and Aulopiformes as well
the total consumption of mesopelagic species increases to between 140 000 and 565 000
tons. These numbers suggest that mesopelagic fish are an abundant and an important prey
source in the Benguela region. According to Shannon and Jarre-Teichmann (1999) about
1.7 106 tons of mesopelagic fish (lanternfish and lightfish) are required among others to
support predators such as hakes of the northern Benguela region.
3.2.5 Life history
Table 2 gives an overview of various life history parameters of selected mesopelagic
families, showing ranges instead of single values for most parameters mentioned. It
becomes apparent that parameters such as maximum length, length infinity, growth
parameters, age at maturity etc. are highly variable within and between families. Making
generalizations based on this table would be difficult. Therefore the information will not
be discussed in detail in the following paragraphs, but merely referred to where
applicable.
Gartner (1993) reported average lifespans of 300 and 375 days for two lanternfish found
in the Gulf of Mexico, while an ageing study of the most abundant lanternfish in the
southern oceans, Electrona antarcticus, suggested a maximum life span of 3.5 years
(Greely 1999). This is fairly short compared to some life spans shown in Table 2. Species
belonging to the Bathylagidae and Argentinidae can grow to be older than 20 years, while
myctophid species' longevity is 0.7 20.2 years. Some species living at higher latitudes
26
BCLME project LMR/CF/03/08
Table 2: Life history data of some selected mesopelagic families. The information shown is a
summary of data obtained from referenced literature listed in FishBase (2005, www.fishbase.org).
The references shown for each family do not contain the data listed in the table, but instead refer
to chapters in Smith and Heemstra (1991) with information on the specific family.
Family
Lmax
(cm)
L
K
t0
M
Tm
Am
Reference
Nemichthyidae
55 57.1 0.19 -1.15 0.21 5.6
160.7 164
0.51
-0.27
0.61
15.2
1.3 3.6
Castle 1991;
Nelson 1994
Argentinidae
7 70 7.5 0.12 -1.22 0.19 2.3
52.5
1.22
-0.19
1.98
23.6
0.8 5.6
Cohen 1991a
Bathylagidae
9.3 9.9 0.11 -2.21 0.24 9.0
26.6
27.9
0.31
-0.69
0.59
25.7
2.7 6.8
Cohen 1991b;
Gon 1990
Platytroctidae
9.3 9.9 0.75 -0.37 0.88 1.2
33
35
2.36
-0.09
2.68
3.8
0.4 1.2
Matsui 1991
Stomiidae
Gibbs 1991a
Chauliodontidae*
Gibbs 1991b
Astronesthidae*
Gibbs 1991c
2.5 2.7 0.15 -1.6
0.27 1.1
Idiacanthidae*
Gibbs 1991d
53
55.1
2.46
-0.12
1.71
19
0.4 4.4
Malacosteidae*
Goodyear &
Gibbs 1991
Melanostomatidae*
Gibbs 19912
Photichthyidae
4 30 4.3 1.01 -0.39 1.37 0.4
34.5
7.11
-0.04
6.15
2.8
0.2 1.6
Schaefer et al.
1991
Gonostomatidae
2 36 2.2 0.17 -2.0
0.42 1.4
37.6
1.98
-0.14
3.21
16.7
0.5 6.1
Schaefer et al.
1991
Sternoptychidae
2 14 2.1 0.42 -0.81
0.7 1.3
14.9
2.14
-0.15
1.96
7
0.7 2.6
Weitzman
1991
Scopelarchidae
3.7 4.0
35
36.6
/
/
0.52
/
/
Johnson 1991
Notosudidae
11 11.7 0.31 -0.79 0.43 2.6
50
52
1.06
-0.19
1.19
9.2
0.8 2.6
Krefft 1991
Evermannellidae
18.5
19.5
0.38 -1.08
0.93
4.6
0.61
-0.32
7.4
1.3 3.4
Johnson 1991
Myctophidae
2.3 2.5 0.17 -1.74
0.42 0.7
30
31.5
3.65
-0.07
6.73
20.2
0.3 5.1
Hulley 1991
Neoscopelidae
20 21 0.26 -1.48
0.63
6.1
30.5
32
0.46
-0.39
10.9
1.7 4.6
Hulley 1991
Lmax maximum length (can be TL or SL, cm); L - length infinity (cm); K curvature parameter (growth rate); t0 initial condition
parameter; M natural mortality; Tm longevity (max age, years); Am age at maturity (years); * In FishBase (2005) these families
are listed as subfamilies of the family Stomiidae.
can become larger and older (Salvanes & Kristoffersen 2001). Still, an ageing study on
the lightfish Electrona antarcticus suggests higher growth rates of mesopelagic species
than previously thought of (Greely 1999), suggesting shorter life spans covering one or a
few years. Generally mesopelagic fish living in warm waters reach their maximum size in
a year or less and grow quicker than species living in cold waters (Gjøsæter &
Kawaguchi 1980). This also seems to be the case with lantern and light fish off southern
Africa (Prosch et al. 1995). The max age of L. hectoris from eastern Tasmania was
estimated at 3 years (Young et al. 1988), similar to that from the southern Benguela
(Prosch 1986). The fish reached a similar maximum size L (7.3cm SL) to those recorded
off South Africa (7cm SL, Olivar et al. 1998), while Gjøsæter & Kawaguchi (1980)
27
BCLME project LMR/CF/03/08
report on a L of 10cm, and a growth rate K of 0.31. Length at age data suggested three
year-classes, and annual mortality of L. hectoris was calculated at 79 % (Young et al.
1988), compared to 50% for B. glaciale, 55% for N. kroeyeri and 83% for M. muelleri
(Gjøsæter & Kawaguchi 1980). For southern African L. hectoris and M. muelleri Prosch
(1986) estimated total annual mortality at 99% and 90% respectively.
An age and growth study of lanternfish from the Gulf of Mexico puts the age at sexual
maturity at 140 and 180 days for B. suborbitale and Lepidophanes guentheri respectively
(Gartner 1991). Length at sexual maturity (Lm) was estimated at 2.3 and 4.3cm
respectively for these two species, while other lanternfish Lm ranged from 1.7 to 5.5cm
(Gartner 1993). In the southern Benguela length at first maturity for both L. hectoris
sexes was 3.6cm Lc, while 2.4 and 2.6cm Lc for male and female M. muelleri respectively
(Prosch 1991).
Crawford (1980) described the length weight relationship parameters for L. hectoris as
follows:
W = 0.0242L 2.6838
c
r = 0.96
n = 800
L. hectoris can reach 7 to 8 cm in standard length (SL) (Crawford 1980; O'Toole 1976;
Olivar et al. 1998), while some Symbolophorus species reach 7.5 cm, but can grow to be
between 12 and 16cm in length (O'toole 1976; Prosch et al. 1995). Based on incidental
catches off Namibia, O'Toole (1976) lists standard lengths of several other mesopaelagic
species. The maximum recorded length of the hatchetfish M. muelleri was 3.5 cm, of the
scaly dragonfish Stomias boa boa 13 cm and of two bristlemouth species 4.2 cm.
Many mesopelagic fish species exhibit sexual dimorphism, with usually females being
larger in size than male fish. M. muelleri and L. hectoris females for example are larger in
size than their male counterparts (Hulley & Prosch 1987; Prosch 1991). The modal length
of male L. hectoris was 4.3 cm compared to between 5.6 and 5.9 cm for females (Hulley
& Prosch 1987). Possible explanations for this are a lower mortality and/or higher growth
rates among females (Salvanes & Kristoffersen 2001).
The fecundity of mesopelagic fish is as a result of their small size generally low. Yet they
still have a higher reproductive rate than long-lived epipelagic species, which have a
higher fecundity (Salvanes & Kristoffersen 2001). The average absolute fecundity of the
myctophid P. choriodon, found in the Southwest Atlantic, was 84400 eggs (with an
average length of 8.5 cm; Oven 1990). The females of this species lay an average 8900
eggs in a batch (Oven 1990). Young et al. (1987) reported fecundities for L. hectoris and
M. muelleri off Australia that ranged between 1309 2798 and 104 942 respectively.
Off southern Africa the fecundity of L. hectoris ranged from 571 to 1431, with a mean of
646 eggs/g wet body mass. M. muelleri fecundity varied between 161 and 738, and had
an average of 334 eggs/g wet body mass (Prosch 1991).
3.2.6 Spawning and early life stages
Many lantern and lightfish do not seem to grow older than a few years, implying that in
this time period the fish must mature and spawn. Some species are batch spawners, and
spawn repeatedly throughout an extended season spanning several months. The produced
28
BCLME project LMR/CF/03/08
eggs do not differ from those of pelagic fish, and range in diameter between 0.5 and 1.65
mm (Salvanes & Kristoffersen 2001). In temperate and sub-tropical regions, peak
spawning of lanternfish seems to coincide with periods of high zooplankton abundance
from late winter to early summer (Gjøsæter & Kawaguchi 1980; Prosch et al. 1995;
Sabatés & Olivar 1989).
Sabatés and Olivar (1989) provide an overview of fish larvae species (Order
Stomiiformes and Myctophiformes) identified in plankton samples collected between
1979 and 1986 in the northern Benguela (17030'S 29030'S; also see Olivar & Shelton
1993 for distribution maps). They found that larvae of the pseudo-oceanic species L.
hectoris and M. muelleri were more abundant than for example the more oceanic
Symbolophorus species. This confirms the findings by O'Toole (1977), who reports that
L. hectoris and M. muelleri larvae were more numerous than Symbolophorus species
larvae. Peak L. hectoris and M. muelleri larval abundance was recorded during the main
upwelling season winter and spring months, with the main spawning areas located
beyond the 150 200 m isobath (O'Toole 1977; Olivar & Shelton 1993; Sabatés &
Olivar 1989). Similarly high densities of L. hectoris larvae were found outside the
Lüderitz upwelling area and north above the continental slope, between the 500 and
1000m isobath and beyond (Olivar et al. 1998). Olivar et al. (1998) also describe high
densities of L. hectoris larvae further offshore than the 4000m isobath. They suggested
that unusually long upwelling filaments, as mentioned in Shannon (1986), transported
these larvae away from their usual spawning area. According to Olivar et al. (1992) more
than 70% of L. hectoris eggs and larvae are located in the upper 100m. Mean number of
L. hectoris eggs and larvae during winter and spring months was 1.416 and 878 10m-2
(Olivar & Shelton 1993).
Prosch (1991) gives an overview of existing data on the reproductive biology of L.
hectoris and the hatchet fish M. muelleri in the southern Benguela. In the study egg and
larval data collected in the period August 1977 to August 1978 was analysed. Temporal
distributions indicated peak spawning between July/August and October for both species,
with prolonged M. muelleri spawning occurring also in November (Hulley & Prosch
1987; Prosch 1991; Olivar & Shelton 1993). No gonad activity was observed between
December and March (Hulley & Prosch 1987). Highest densities of L. hectoris eggs and
larvae were observed during maximum spawning in August, offshore of the 200m isobath
off Cape Columbine and Cape Peninsula. Average densities of eggs and larvae were 170
and 667 10m-2 (Olivar & Shelton 1993), compared to moderate densities of 110
1000 10m-2 reported by Prosch (1991).
John and Kloppmann (1993) give a detailed description of the vertical distribution of M.
muelleri eggs in the northern Benguela. Eggs of this species occurred from the surface to
a depth of at least 200m. The maximum number of eggs was recorded between 25 and 60
m depth and this egg maximum occurred at a temperature range of 13-140C. The mean
abundance of eggs was 555 10m-2, and for larvae 339 10m-2 (Olivar & Shelton 1993).
In the southern Benguela M. muelleri eggs were present closer inshore than lanternfish
eggs. Highest densities followed the 200 m isobath and were observed between Cape
29
BCLME project LMR/CF/03/08
Columbine and Cape Town, as well as the western edge of the Agulhas Bank. Moderate
densities of M. muelleri larvae (110-1000 10m-2) stretched northwards from the Cape
Peninsula to approximate 31.50S (Prosch 1991). Similarly Olivar and Shelton (1993)
report egg and larvae abundances of 734 and 107 10m-2 respectively off southern Africa.
Eggs of M. muelleri were most abundant at 0 40m depth, with fewer found deeper then
100m (John & Kloppmann 1993). More on M. muelleri ichthyology can be found in
Prosch (1991) and John and Kloppmann (1993).
The myctophid S. boobs was observed to spawn over the slope in the southern northern
Benguela towards the Orange River (Olivar 1990). The larvae of this species had the
highest density at 20-40m depth. Olivar and Beckley (1994) provided additional
information on the distribution and morphometrics of other Symbolophorus species off
Namibia, while Olivar (1987) described the larval development and some spawning
characteristics of the lanternfish D. hudsoni in the area between 17030'S and 350S. D.
hudsoni larvae were most abundant in an area around 200S and between 290 and 300S, in
waters with bottom depth greater than 400m. With the exception of autumn, spawning of
this species takes place during most of the year. The presence of larvae as far as 300km
offshore suggests that this species also spawns in oceanic waters (Olivar, 1987).
O'Toole (1977) reported on the relative abundance of a Chauliodus spp.
(Chauliodontidae) and Stomias boa boa (Stomiidae) larvae during SWAPEL surveys
from 1972-1974. Since only few specimens of these two families were caught no
additional information was provided.
3.2.7 Abundance
The lanternfish have the highest abundance of all organisms in the mesopelagic zone,
while their larvae make up the biggest part of the vertebrate plankton abundance (Prosch
et al. 1995).
Research done in the North-east Atlantic suggests that abundance of mesopelagic fishes
varies depending on bottom topography and bathymetric depth. Higher biomasses were
observed in oceanic deep water compared to both seamount slopes and plateaus (Pusch
2002). Williams & Koslow (1997) observed a marked day/night shift in micronekton
distribution off Tasmania. During the day 0.2% of the micronekton biomass, dominated
by myctophids, was found shallower than 300m, while during nightime 53% of the
biomass was found above 300 m. May and Blaber (1989) suggested that seasonal peaks
in abundance of mesopelagic fish, of which L. hectoris made up >90%, was linked to
seasonal cycles in water temperature, nutrients and primary productivity. The seasonal
increases in abundance they argued could be related to the southward penetration of the
East Australian current.
An estimate of abundance of mesopelagic fish for the entire Benguela region is not
available, but a number of abundance estimates have been calculated for the southern
Benguela current region and the south-eastern Atlantic Ocean for some mesopelagic fish
families or species.
30
BCLME project LMR/CF/03/08
Shelton and Davis (1979) first suggested the existence of large L. hectoris and M.
muelleri populations over the southern Benguela shelf, while Cruickshank et al. (1982)
reported on substantial stocks of lanternfish in the northern Benguela. Based on acoustic
abundance data from 1983 and 1987, as well as egg abundance and catch rate data,
Armstrong and Prosch (1991) estimated mean densities of M. muelleri in the southern
Benguela ranging from 4 to 10t km-2. Using a mean of 7.41t km-2, the total biomass for
the surveyed region was 547 900mt, and extrapolating this to the un-surveyed northern
Benguela region gives about 1 106 tons of lightfish (Armstrong & Prosch, 1991). Based
on SWAPELS1 data from 1978/79 to 1983, Hewitson and Cruickshank (1993) estimated
the abundance of lanternfish in the northern Benguela (between 150 and 290S and inshore
to 500m bottom depth) at 0.4 106mt. This estimate was based on incidental catches of fish
in bongo nets, and was subsequently corrected for biases such as net avoidance and under
sampling during the day due to vertical migration of the fish. A correction factor of two
was applied and the estimate adjusted to 0.8 106mt. Hulley (1986) estimated the offshore
stock of myctophids in the eastern south Atlantic (28040'S to 40000'S) at between 8 106
and 12 106mt, depending on the gear and cruise data used. This is approximately 50-70%
of the entire mesopelagic stock estimated to be 18 106mt for the eastern south Atlantic
(Gjøsæter and Kawaguchi, 1980). In comparison, acoustic estimates of mesopelagic fish
in specific areas off New Zealand ranged from 0.44 106mt to 0.67 106mt (McClatchie,
2003).
3.2.8 Catch history
L. hectoris is the only mesopelagic fish species that has been targeted and caught in
reasonable amounts by the purse seine fishery in South Africa (Crawford 1980; Crawford
1987). This species has also been recorded as by-catch in Namibian waters. During the
1979 season for instance 1141mt of lanternfish were caught (Cruickshank 1982).
Although catches of lanternfish in Namibian waters have been recorded, no official catch
statistics are available. Statistics of the pelagic goby and L. hectoris catches made in
South African waters are listed in Annex 14.
Crawford (1980) gives a detailed description of the occurrence and distribution of L.
hectoris catches by the South African purse-seine fishery between 1968 and 1976. During
this period the majority of catches were made between Olifants River and Dassen Island,
with highest availability in January and February (no fishing was allowed between
September and December). Catches were usually processed to fishmeal, although the
high oil content of mesopelagic species (mostly L. hectoris) turned out to be a major
problem for the industry by clogging the fishmeal machinery (D.Boyer, pers. com.;
Crawford 1987; Hulley & Prosch 1987). In the 90's attempts to target mesopelagics in
Namibian waters were thwarted again due to similar previously experienced processing
problems (D.Boyer, pers. com).
31
BCLME project LMR/CF/03/08
3.3 Regional data sources
3.3.1 Nan-Sis
A total of 146 demersal and pelagic surveys done in the Benguela current region and
captured in the Nan-Sis database have been identified and queried for the purpose of this
report (Table 3).Between 1985 and 2005 a total of 47, 51 and 8 country specific surveys
were conducted in Angolan, Namibian, and South African waters respectively. Of the
Namibian surveys queried the data of eight surveys (NC) were collected onboard
commercial fishing vessels. In addition 37 multi disciplinary and multi national
BENEFIT surveys have been completed in the region since 1997 (Table 3). The data
gathered during the time period of the surveys was collected on various vessels, i.e. the
old and new RV Fridtjof Nansen and various commercial fishing vessels. For comparison
purposes the fishing gear specifications were identical on each of the vessel used. In the
Namibian projects searched no records of members of the families Platytroctidae,
Scopelarchidae, and the Evermannellidae could be found. Likewise records of the
Idiacanthidae, Scopelarchidae, Evermannellidae, and the Neoscopelidae were absent in
the Angolan projects (Table 4). The total number of stations found was highest in
Namibia, followed by Angola, South Africa and the BENEFIT project (Table 5). There
was considerable variation between the numbers of stations per family identified, from as
few as one to 1820. Based on the ranking of the five most frequently found families,
Angola and Namibia had four families in common, Angola and South Africa two, and
Namibia and South Africa three (Table 6).
Table 3: Summary of project related surveys captured in the Nan-Sis database.
Country /
Organization
Project code Year / Period
# of surveys Station # range
Angola
AN
1985 1986
6
1 992
A2
1989
3
1 531
A3
1991 1993
6
1 838
A4
1994 2005
29
1 3896
NA
1992
1
1276 1297
N1
1996
1
1646 1662
BE
1997
1
1257 1285
Namibia
NA
1990 1993
15
1 2090
N1
1994 1998
22
1 2457; 2506 2595
N2
1999 2002
6
2596 2980
NC
1998 2005
8
2231 2442; 2600
4047
South Africa
SA
1994 1995
3
1 27
2000 2001
4
28 385
2004
1
903 949
BCLME
SA
2004 2005
2
806 855; 950 1108*
BC
2004
1
865 902
BENEFIT
BE
1997 2005
33
1 80; 128 1552
SA
2002 2005
4
386 805; 1109 1151*
* SA project codes under BENEFIT & BCLME are found in the database under the SA project code, i.e. the station range found in the SA project is from 1 1151.
32
BCLME project LMR/CF/03/08
Table 4: Orders and families searched for in the Nan-Sis database. Tick marks indicate that
members of a certain family were found in any one of a countries projects, while a cross indicates
the absence.
Country (projects)
Order
Family
Angola
Namibia
South Africa
Argentiformes
Argentinidae
Bathylagidae
Platytroctidae
x
Stomiiformes
Stomiidae
Chauliodontidae
Astronesthidae
Idiacanthidae
x
Malacosteidae
Photichthyidae
Gonostomatidae
Sternoptychidae
Melanostomatidae
Aulopiformes
Scopelarchidae
x
x
Notosudidae
Evermannellidae
x
x
Myctophiformes
Myctophidae
Neoscopelidae
x
Anguilliformes
Nemichthyidae
Perciformes
Gobiidae
Table 5: Total number of stations for mesopelagic families and Gobiidae stored in Nan-Sis. The
total number of stations by country is the sum of stations identified in that countries respective
projects, e.g. NA, NC, N1 and N2 are projects linked to Namibia (refer to Table 3).
Country
Project
Order
Family
Angola
Namibia
South Africa BENEFIT
Argentiformes
Argentinidae
2
3
9
---
Bathylagidae
7
151
15
13
Platytroctidae
8
---
4
---
Stomiiformes
Stomiidae
303
194
8
41
Chauliodontidae
24
16
36
3
Astronesthidae
19
15
19
1
Idiacanthidae
---
3
11
---
Malacosteidae
---
30
31
6
Photichthyidae
416
1044
255
134
Gonostomatidae
694
354
30
54
Sternoptychidae
63
181
317
75
Melanostomatidae
244
80
51
2
Aulopiformes
Scopelarchidae
---
---
9
---
Notosudidae
21
54
65
7
Evermannellidae
---
---
2
---
Myctophiformes
Myctophidae
943
1820
912
418
Neoscopelidae
---
65
28
2
Anguilliformes
Nemichthyidae
199
196
70
9
Perciformes
Gobiidae*
273
1868
98
113
Total
2943
4206
1872
765
*Not included in the total.
33
BCLME project LMR/CF/03/08
Table 6: Percentage of stations per family by country and their ranking. Shaded cells indicate the
five most common families identified in each country.
Country
Project
Order
Family
Angola
Namibia
South Africa
BENEFIT
%
rank
%
rank
%
rank
%
rank
Argentiformes
Argentinidae
0.1
13
0.1
13
0.5
14
Bathylagidae
0.2
12
3.6
7
0.8
12
1.7
6
Platytroctidae
0.3
11
0.2
16
Stomiiformes
Stomiidae
10.3
4
4.6
5
0.4
15
5.4
5
Chauliodontidae
0.8
8
0.4
12
1.9
7
0.4
10
Astronesthidae
0.6
10
0.4
12
1.0
11
0.1
12
Idiacanthidae
0.1
13
0.6
13
Malacosteidae
0.7
11
1.7
8
0.8
9
Photichthyidae
14.1
3
24.8
2
13.6
3
17.5
2
Gonostomatidae
23.6
2
8.4
3
1.6
9
7.1
4
Sternoptychidae
2.1
7
4.3
6
16.9
2
9.8
3
Melanostomatidae
8.3
5
1.9
8
2.7
6
0.3
11
Aulopiformes
Scopelarchidae
0.5
14
Notosudidae
0.7
9
1.3
10
3.5
5
0.9
8
Evermannellidae
0.1
17
Myctophiformes
Myctophidae
32.0
1
43.3
1
48.7
1
54.6
1
Neoscopelidae
1.5
9
1.5
10
0.3
11
Anguilliformes
Nemichthyidae
6.8
6
4.7
4
3.7
4
1.2
7
The Myctophidae was the most frequently observed family in the region (Table 6). The
percentage of stations with Myctophidae ranged from 32% (Angola) to 54.6%
(BENEFIT). With regard to the Angolan data many of the myctophids were only
identified to the family level. When identification was done to genus or species level, the
dominant genus was Diaphus and the dominant species Diaphus dumerili (Appendix 4,
Table A). In Namibia and South Africa the most frequently identified lanternfish were L.
hectoris and S. boobs (Appendix 5, Table A; Appendix 6, Table B). The distribution map
for this family shows very clearly the prominent presence over the shelf and slope along
the entire southwest African coast (Appendix 12, Table 1A). Average depths where
various species of this family were caught ranged from 325-595m bottom depth and from
280-565m fishing depth.
The other family abundant in all three countries was the Photichthyidae (lightfish),
ranking at either two or three (Table 6). In contrast to the distribution of the Myctophidae,
fish from this family occurred in deeper waters on the continental slope (Appendix 10,
Figure 1B), with an average bottom and fishing depth of 530 and 515m. The species
Yarella blackfordi was identified most frequently in Angola and Namibia (Appendix 4,
Table B; Appendix 5, Table A), while Photichthys argentus was the dominant lightfish
species in South Africa (Appendix 6, Table B).
Stomias boa boa (Stomiidae) was the most abundant of the scaly dragonfish in Angolan
and Namibian waters, ranked 4th and 5th respectively (Appendix 4, Table B; Appendix 5,
Table B). Ranking at 15th place and also apparent from the distribution map, this family
was less abundant in South African waters (Appendix 9, Figure 1A). In Namibia and
Angola the average bottom depth where this family was taken was 560 m.
34
BCLME project LMR/CF/03/08
Also abundant both in Namibia and Angola, but mainly only identified to genus level in
Namibia, were the bristlemouths (Gonostomatidae). The most prevalent member of this
family, which ranked as the 2nd most abundant in Angola, was Triplophos hemingi
(Appendix 4, Table A). The depth distribution was similar to that of the scaly dragonfish,
averaging at 560 m bottom depth. The distribution map for this family shows the patchy
presence in the southern Benguela, and the obvious absence between 13 and 170S
(Appendix 10, Figure 1C). It needs to be mentioned here that the general absence in this
region is not due to the actual physical absence of the families, but the lack of data. The
reason for this is that along this stretch the shelf is very narrow, and the sampling density
thus was much lower here than anywhere else.
The Melanostomatidae (scaleless dragonfish), which ranked 6th and 8th in Namibia and
South Africa respectively, was the 5th most common family in Angolan waters, with
Melanostomias the dominant genus (Appendix 4, Table A). The major Nemichthyidae
(snipe eel) genus observed was Nemichthys and ranked 4th both in Namibia and South
Africa (Appendix 5, TableA, Appendix 6, Table B).
M. muelleri (Sternoptychidae) ranked 2nd in South Africa, compared to 7th and 6th in
Angola and Namibia respectively (Table 6). This species often referred to as a lightfish
but classified as a hatchetfish, showed a broad distribution in the southern Benguela
(Appendix 10, Figure 1D). Although the average bottom at which this species was caught
was app. 340 m, it seems that it occurs both on the shelf and further offshore on the slope.
Members of the family Evermannellidae (sabretoothed fishes) were never or rarely
identified, with no records from Angola and Namibia, and only two stations in South
African waters. Also infrequent (<0.6 % of total number of stations) in all three countries
were the families Argentinidae (argentines), Platytroctidae (tubeshoulders), (pearleyes),
and Idiacanthidae (sawtail fishes; Table 6)
3.3.2 MCM data
A total of 50 surveys were performed by the RV African between 1985 and 2004 (Table
7). Some 4664 stations were sampled out of which 2274 contained one or more
mesopelagic fish species (Appendix 6, Table D G). The most frequently sampled family
was the myctophids, followed by the hatchetfishes, lightfishes, snipe eels, and viper
fishes (Table 8). The ranking of the families recorded was nearly identical to that of the
data from the Nansen, with the only difference that instead of the notosudids the viperfish
ranked 5th (Table 8).
3.3.3 NatMIRC data
Very little data except for the data captured in Nan-Sis during the annual demersal
abundance estimate surveys with commersial vessels was available from NatMIRC.
Surveys with the RV Welwitchia have however regularly collected information on gobies
and mesopelagic species as part of the abudance surveys of horse mackerel, sardine and
monk. The data are however scattered and not in a centralized database and therefor
difficult to obtain easily. An overview of the origin and location of some of the
35
BCLME project LMR/CF/03/08
mesopelagics data is given in Table 9, while the location of goby and mesopelagic by-
catches during the annual horse mackerel surveys is shown in Annex 13.
Table 7: Summary of relevant surveys conducted by the RV Africana in the period from 1985 to
2004.
Year
Cruise #
# of surveys
# of station
1985
C028, C033
2
196
1986
C039, C046, C048
3
300
1987
C050, C054, C056
3
300
1988
C059, C063, C066
3
311
1989
C069, C072, C075
3
231
1990
C079, C082, C084, C086
4
354
1991
C088, C093, C095
3
292
1992
C100, C102, C106
3
296
1993
C109, C111, C116
3
326
1994
C118, C122, 125
3
319
1995
C127, C129, C131
3
322
1996
C133, C135
2
186
1997
C139, C144
2
203
1999
C150, C152
2
171
2001
C160
1
80
2002
C161
1
47
2003
C173, C177, C182
3
273
2004
C188,C191,C200
3
291
Table 8: Total number of stations, percentages and ranking of mesopelagic families and the
Gobiiodae stored in the Africana Demersal Data System.
Order
Family
# stations
% stations RANK
Argentiformes
Argentinidae
2
0,8
9
Bathylagidae
19
0,7
10
Platytroctidae
10
0,4
12
Stomiiformes
Stomiidae
13
0,5
11
Chauliodontidae
64
2,3
5
Astronesthidae
18
0,7
10
Idiacanthidae
22
0,8
9
Malacosteidae
40
1,5
7
Photichthyidae
313
11,5
3
Gonostomatidae
29
1,1
8
Sternoptychidae
777
28,5
2
Melanostomiidae
48
1,8
6
Aulopiformes
Scopelarchidae
11
0,4
12
Notosudidae
49
1,8
6
Evermannellidae
2
0,1
13
Myctophiformes Myctophidae
1237
45,4
1
Anguilliformes
Nemichthyidae
70
2,6
4
Perciformes
Gobiidae*
313
TOTAL
2724
*Not included in the total.
36
BCLME project LMR/CF/03/08
Table 9: Overview of mesopelagic data availability from Namibian waters.
Activity
Vessel / Source
Species / Fishery
Period
Comments
Research
RV Benguela
Pelagic
1980's - early
No records of by-catch captured
surveys
1990's
electronically. Hard copies contain
some by-catch records.
RV Nansen
All
Annex 5, project codes NA, N1
and N2
RV Welwitchia
Horse mackerel -
1999 present
pelagic
(February)
Trawl by-catch data captured in an
access database. Records are
registered at species and family
level, i.e. gobies, myctophids etc
Pilchard - pelagic
Mar/Apr and
October
Same as above
Monk - demersal
2000 Present
(November)
Limited mesopelagics data
FV Blue Sea
Hake - demersal
2001 Present
(Jan/Feb)
Annex 5, project code NC
Commercial Logsheet
Horse mackerel
1997 present No records of mesopelagics. Mesh
fishing
midwater fishery
size > 60 mm. Maybe occasionally
caught and possibly processed to
fishmeal.
Logsheet
Horse mackerel,
1997 present Some bycatch data of
pelagic fishery
pilchard, anchovy
mesopelagics captured. Available
in an access database.
Historical
Midwater
Before 1997
Catch logs contain no records of
mesopelagics
Pelagic
From early
ICSEAF records contain data on
1970's
all species caught in the ICSEAF
(1973 1989)
area, including records on
mesopelagics Lampanyctodes
hectoris, and gobies. These are
listed but not available in
electronic format.
3.4 Ongoing and recently completed research projects
BENEFIT / BCLME Project No. N01/017
Project Name: Biology and Ecology of Pelagic Gobies
Duration of Project (2001 - 2005) - Cruises conducted in 2003, 2004, 2006.
This project was originally proposed, and funded, in 2000 with the goal of improve the
understanding of the feeding biology and of the abundance of S. bibarbatus off Namibia.
One should as part of the project investigate ways to develop a methodology suitable for
estimation of biomass and distribution of S. bibarbatus off Namibia. The project has been
through several iterations in order to allow for an overlap with a project entitled "The
Ecology of Gobies in the Benguela Ecosystem" that was successfully submitted to the
37
BCLME project LMR/CF/03/08
South Africa Norway Programme on Research Cooperation for funding in 2003. The
latter project is described in more detail below. The BENEFIT projects level of support as
a consequence shifted from one essentially based on the provision of running expenses, to
one based largely on ship's time.
The project is essentially being used as a student training exercise and it has been
constructed around a number of small, discrete studies. The students include MSc
students from RSA that are studying in RSA, and MPhil students from the region
studying in Norway. It also includes Norwegian and other students studying at the MPhil
and PhD level in Norway.
Original Objectives
· Improve our understanding of the feeding biology of S. bibarbatus off Namibia
· Improve our understanding of the biomass and distribution of S. bibarbatus off
Namibia
Additional Objectives arising from the SA-Norway Programme on Research Co-
operation
· Improve our understanding of the behaviour of S. bibarbatus off Namibia, with
particular reference to diel vertical migration and tolerance of low oxygen water
NORWAY-SOUTH AFRICA RESEARCH COOPERATION Norwegian Research
Council Project No: 152309/v10
Project Name: The Ecology of Gobies in the Benguela Ecosystem
Duration of project (2003-2006)
The project essentially expanded the aims to include more focused studies on the food
and feeding, behaviour and physiology of S. bibarbatus.
Objectives: Investigate the feeding ecology, and reproductive and population biology of
S. bibarbatus and its role of the Benguela ecosystem by
· Study diet and feeding biology of S. bibarbatus
· Study population biology of S. bibarbatus.
MASTER PROGRAM STUDIES
The following three students have all received higher degrees through data collected, in
part, through the BENEFIT goby project and the Norway-South Africa research
cooperation on gobies. All students received bursary funding through the NORAD
programme: the first two students are South Africans and were based at M&CM in Cape
Town, whilst the third comes from Sri Lanka.
Shipokazi Nduane (2004) Population genetic studies of S. bibarbatus in the Benguela
ecosystem M.Phil. University of Bergen, Norway. Supervisor: Prof AGV Salvanes
38
BCLME project LMR/CF/03/08
Rengque Judy Lungelwa (2005) Diel Vertical Migrations of the Pelagic goby S.
bibarbatus in the Northern Benguela ecosystem M.Phil. University of Bergen, Norway.
Supervisor: Prof AGV Salvanes and MJ Gibbons.
Mangala Pallgae de Silva (2005) Population genetic structure of the pelagic goby, S.
bibarbatus, in the Northern Benguela Ecosystem, based on PCR, RFLP analysis of the
mitochondrial control region and the ND ¾ region M.Phil. University of Bergen,
Norway. Supervisor: Prof AGV Salvanes
IN PROGRESS
Anthony, KA Studies on the diet and feeding of the pelagic goby, Sufflogobius
bibarbatus. MSc Thesis, University of the Western Cape, South Africa. Supervisor:
Professor MJ Gibbons.
Staby, A. Investigating swimming dynamics and feeding behaviour of mesopelagic fish in
the Benguela current system and a Norwegian fjord using acoustics, PhD studies in
progress, University of Bergen, Norway. Supervisors: Professor Anne Gro Vea Salvanes
and Professor Stein Kaartvedt from the University of Oslo. This will be a comparative
study of behaviour, distribution and biology of mesopelagic species in the Benguela
system and a Norwegian fjord system.
39
BCLME project LMR/CF/03/08
4 Summary
Gobies
Given that pelagic gobies together with the mesopelagic fish resources are currently of
the few unexploited resources in the region that could be sufficiently numerous to have
the potential for future exploitation, it is likely that regional governments may allow, or
even encourage, a targeted goby fishery. The increased predation pressure, coupled with
any focussed fishery, is likely to have a severe influence on the population dynamics and
production of gobies, and research has been needed to determine the knowledge
available on this resource and identify gaps where more research is needed.
The species is abundant in the region and a key species in the diet of many predator fish
species, seals and seabirds. But the stock is possibly not as abundant as some authors
have suggested. Data collected from swept area surveys of Namibia with RV Dr. Fridtjof
Nansen suggests that the stock is much smaller than previously reported. The index of
abundance presented in this report does however underestimate the stock to some extent.
The report from the BENEFIT Project No. N01/017 suggests that acoustic abundance
estimation is not suitable for gobies with today's technology, and it may be difficult to
find any reliable direct way of estimating the entire stock.
There is still a lack of knowledge regarding the life history parameters of the species
although a resent study (Melo and Le Clus 2005) has expanded our knowledge in this
field. The distribution maps presented in this report are the most extensive and up-to- date
available and has not previously been published. We should now have a complete
overview of the distribution area of the species although more research is needed on
seasonal dynamics, and spatial and temporal variations in fish size distribution. Two
different populations /subpopulations have been suggested from genetic studies and more
knowledge is needed on the distribution and overlap of these. There is no knowledge on
where the spawning area(s) for the species can be found and on how the early life stages
cope with the hostile conditions on the Benguela shelf. There is also a question on what
physiological adaptations adult gobies have developed to cope with the anoxia and high
sulphur concentrations on the shelf. These questions should be looked into in future
studies.
Mesopelagics
The amount of knowledge on any of the aspects covered in this report with regard to the
species L. hectoris and M. muelleri is much greater than that of any of the other
mesopelagic families found in the Benguela current region. These two species seem to be
the most abundant in the region, based on previous abundance estimates, which might
also explain the amount of information available on them. Yet, there are still gaps with
regard to life history parameters, their ecology and trophic dynamics.
Little if any information is available on mesopelagic families other than the Myctophidae
and the Sternoptychidae (Table 8). Some species, like Yarella blackfordi and Photichthys
argenteus (Photichthyidae) are widely distributed and abundant in trawl catches from the
Benguela region. Little however is known about their biology, ecology, vertical
40
BCLME project LMR/CF/03/08
Table 8: Summary of available information on the most common mesopelagic families in the
Benguela region.
Mycoph.*
Sternopty.**
Photich.
Gonostom.
Stomiidae
Other
Species diversity
+
+/-
+/-
+/-
+/-
+/-
Species distribution
+
+/-
+/-
+/-
+/-
+/-
Biology
+
+
-
-
-
-
Ecology
+
+
-
-
-
-
Life history
+
+
-
-
-
-
Spawning & early
+
+
-
-
-
-
life stages
Abundance
+
+
-
-
-
-
Catch history
+
-
-
-
-
-
*L.hectoris; ** M. muelleri
distribution and behaviour, life history parameters, or abundance. The same can be said
about Stomias boa boa (Stomiidae) and the bristlemouth fish Triplophos hemingi
(Gonostomatidae).
4.1 Recommendations on future studies
A proposal for a BENEFIT survey focusing on the distribution of mesopelagics between
Walvis Bay and Cape Town, and aiming at providing a possible abundance index of
mesopelagic fish, based on acoustic data, has been submitted to BENEFIT. This survey
would present an opportunity to collect relevant environmental, biological, ecological and
acoustic data on the various mesopelagic species, in addition to data on the more common
species like L. hectoris, M. muelleri, and Diaphus as well as Symbolophorous species.
The proposal for this survey is attached in Appendix 15.
The lack of data and information on mesopelagic fish species from the northern Benguela
extending into Angolan waters is obvious and needs to be addressed. Though some
limited data is available on the distribution of mesopelagic species, of which, like the
lightfish and myctophids, seem to be fairly abundant, virtually little or nothing is know
about these species' life history parameters, ecology, trophic interactions, or abundance.
No proposals for future studies on S. bibarbatus are known to date but it is expected that
the work currently undertaken in the before mentioned research projects funded by
BENEFIT and by the Norway-South Africa Research Cooperation Initiative will lead
towards applications for further research. Such research initiatives should focus on topics
mentioned in the summary of this paper, namely; spawning areas, adaptations of all life
stages to hypoxic conditions and high sulphur concentrations in the water column.
Improving the knowledge on the abundance of the goby and how to assess the stock. It is
important that the suggested proposal for a survey on the mesopelagic species also collect
distribution and abundance data on egg and larvae of the goby resource. There is still a
need to find suitable methods to estimate the abundance of gobies in the water column
and information from the suggested cruise may aid in reaching this goal.
41
BCLME project LMR/CF/03/08
5 References
5.1 Gobies
Andronov, V.N. (1983) Feeding of cape horse mackerel (Trachurus trachurus capensis )
and cape hake (Merluccius capensis ) off Namibia in January 1982. Collect Sci
Pap ICSEAF 10: 1-6
Assorov, V.V., and Kalinina, M.I. (1979) Some peculiarities of the feeding habits of cape
hake and South African deep-water hake. Collect Sci Pap ICSEAF 6: 219-227
Barange, M., and Pillar, S.C. (1992) Cross-shelf circulation, zonation and maintenance
mechanisms of Nyctiphanes capensis and Euphausia hanseni (Euphausiacea) in
the northern Benguela upwelling system. Cont Shelf Res 12: 1027-1042
Barange, M., Hampton, I., Pillar, S.C., and Soule, M.A. (1994) Determination of
composition and vertical structure of fish communities using in situ measurements
of acoustic target strength. Can J Fish Aqauat Sci 51: 99-109
Barber, R.T., and Haedrich, R.L. (1969) Gobies associated with a scattering layer off
South West Africa. Deep-Sea Res Oceanogr I 16: 105-106
Chapman, P., and Shannon, L.V. (1985) The Benguela ecosystem. Part II. Chemistry and
related processess. Oceanogr Mar Biol,Annu Rev 23: 183-251
Chlapowski, K. (1977) Food and feeding of hake in Southwest African seas. Collect Sci
Pap ICSEAF 4
Crawford, R.J.M., Shannon, L.V., and Pollock, D.E. (1987) The Benguela ecosystem.
Part 4. The major fish and invertebrate resources. Aberdeen University Press,
Aberdeen
Crawford, R.J.M., Cruickshank, R.A., Shelton, P.A., and Kruger, I. (1985) Partitioning of
a goby resource amongst four avian predators and evidence for altered trophic
flow in the pelagic community of an intense, perennial upwelling system. S Afr J
Mar Sci: 215-228
Cruickshank, R.A., Cooper, J., and Hampton, I. (1980) Extension to the Geographical
Distribution of Pelagic Goby Sufflogobius bibarbatus Off South West Aftica and
Some Mensural and Energetic Information. Fish Bull (SAfr): 77-82
D'Arcangues. C. (1976) Les couches diffusantes sonores au large du sud-ouest africain.
These presentée à L'Université de Paris VI Pour l'obtention du diplome de
Docteur de 3e cycle
42
BCLME project LMR/CF/03/08
De Silva, P.M.C.M. (2005) Population genetic structure of the pelagic goby, Sufflogobius
bibarbatus, in the Northern benguela Ecosystem, based on PCR, RFLP analysis
of the mitochondrial control region and the ND ¾ region. M.Phil. thesis,
University of Bergen: 48 pp.
Duffy, D.C., Wilson, R.P., and Wilson, M.P. (1987) Spatial and temporal patterns of diet
in the Cape cormorant off southern Africa. Condor 89: 830-834
Froese, R., and Pauly, D. (2005) FishBase Version 11/2005. World Wide Web electronic
publishing
Gibbons, M.J., Goosen, A.J.J., and Wickens, P.A. (2002) Habitat use by demersal nekton
on the continental shelf in the Benguela ecosystem, southern Africa. Fish Bull
100: 475-490
Gibbons, M.J., et al. (2000) Video observations on the habitat association of demersal
nekton in the midshelf benthic environment off the Orange River, Namibia. S Afr
J Mar Sci 22:1-7
Goosen, A.J.J., Gibbons, M.J., and Wickens, P.A. (2000) Underwater observations from
the research submersible Jago on the nekton and nekton communities off the
Orange River mouth. 10 Southern African Marine Science Symposium (SAMSS
2000): Land, Sea and People in the New Millennium, Wilderness
Hamukuaya, H., Bianchi, G., and Baird, D. (2001) The structure of demersal assemblages
off Namibia in relation to abiotic factors. S Afr J Mar Sci 23: 397-417
Hewitson, J.D., and Cruickshank, R.A. (1993) Production and consumption by
planktivorous fish in the northern Benguela ecosystem in the 1980s. S Afr J Mar
Sci 13: 15-24
Hoese, D.F. (1991) Family Gobiidae. In: Smith, M.M., and Heemstra, P.C. (Eds). Smith's
Sea Fishes. Southern Book Publishers, Johannesburg: pp 774-807
Konchina, Y.V. (1986) Distribution and feeding of South African horse mackerel and
hake in the Namibian shelf waters. Collect Sci Pap ICSEAF 13: 7-18
Krakstad, J.-O., et al. (2006) Diel vertical migration in gobies: 10-21 January 2006 (in
preparation).Cruise reports 'Dr Fritjof Nansen' Benefit surveys.1/2006. IMR,
Bergen:
Kuderskaya, R.A. (1985) Largeeye dentex, Dentex macrophthalmus (Bloch) (fam.
Sparidae) in the Southeast Atlantic: Biology and fishery. Collect Sci Pap ICSEAF
12: 95-97
43
BCLME project LMR/CF/03/08
Le Clus, F., du Plessis, S.E., Helmke, K., and Hennig, O. (2002) Cross-shelf and along-
shore distribution of young pelagic goby Sufflogobius bibarbatus in the northern
Benguela. 11 Southern African Marine Science Symposium (SAMSS 2002):
Currents, Coasts Communities, Swakopmund (Namibia)
MacPherson, E. (1983) Ecologia trofica de peces en las costas de Namibia. 1. Habitos
alimentarios. Reseñ exp cient (Supl Investigación pesq) 11: 81-137
Macpherson, E. (1985) Daily ration and feeding periodicity of some fishes off the coast
of Namibia. Mar Ecol Prog Ser 26: 253-260
Mecenero, S. (2005) The diet of the Cape Fur Seal Arctocephalus pusillus pusillus in
Namibia: Variability and fishery interactions. Doctor of Philosophy thesis,
University of Cape Town: 219 pp.
Melo, Y.C., and Le Clus, F. (2005) Growth and reproduction of the pelagic goby
Sufflogobius bibarbatus off the Orange River, southern Africa. SAfrJMarSci 27:
265-273
Miller, P.J. (1990) Gobiidae. In: Quero, J.C., Hureau, J.C., Karrer, C., Post, A., and
Saldanha, L. (Eds). Check-list of the fishes of the eastern tropical Atlantic
(CLOFETA). UNESCO, Paris
Nduane, S. (2004) Population genetic studies of Sufflogobius bibarbatus in the Benguela
ecosystem. M.Phil. thesis, University of Bergen: 65 pp.
Nelson, J.S. (1994) Fishes of the world. John Wiley & Sons, Inc., New York
O'Toole, M.J. (1976) Incidental collections of small and juvenile fishes from egg and
larval surveys off South West Africa (1972-1974). Fish Bull (S Afr): 23-33
O'Toole, M.J. (1977 ) Investigations into some important fish larvae in the southeast
Atlantic in relation to the hydrological environment. Doctor of Philosophy thesis,
University of Cape Town: 308 pp.
O'Toole, M.J. (1978) Development, distribution and relative abundance of the larvae and
early juveniles of the pelagic goby Sufflogobius bibarbatus (von Bonde) off South
West Africa, 1972-1974. Report. Sea Fisheries Branch, S.Afr.1-28
Olivar, M.P. (1981) Distribution and abundance of ichthyoplankton caught during the
cruise Benguela 1: November 1979. Collect Sci Pap ICSEAF 8: 161-173
Olivar, M.P., Rubies, P., and Salat, J. (1992) Horizontal and vertical distribution patterns
of ichthyoplankton under intense upwelling regimes off Namibia. S Afr J Mar Sci
12: 71-82
44
BCLME project LMR/CF/03/08
Olivar, M.P., Moser, H.G., Hartel, K.E., and Lombarte, A. (1993) Larvae of three species
of Bathylagus of the southern Atlantic. Copeia: 503-513
Prenski, L.B. (1980) The food and feeding behaviour of Merluccius capensis in Division
1.5 (with some observations on Division 1.4). Collect Sci Pap ICSEAF 7: 283-
296
Rengqe, J.L. (2005) Diel Vertical Migrations of the Pelagic goby Sufflogobius bibarbatus
in the Northern Benguela ecosystem. M.Phil. thesis, University of Bergen: 64 pp.
Salvanes, A.G.V., Utne-Palm, A.C., Krakstad, J.-O., Midtøy, F., and Chikilikwa, C.
(2004) Diel vertical migration in gobies: 12 - 18 January 2004.Cruise reports 'Dr
Fritjof Nansen' Benefit surveys. 1/2004. IMR, Bergen
Shannon, L.J., and Jarre-Teichmann, A. (1999) A Model of trophic flows in the northern
Benguela upwelling system during the 1980s. S Afr J Mar Sci 21: 349-366
Villanueva, R. (1993) Diet and mandibular growth of Octopus magnificus (Cephalopoda).
S Afr J Mar Sci 13: 121-126
Von Bonde, C. (1923) Shallow-water fishes procured by the S. S. "Pickle". Fisheries and
Marine Biological Survey, Pretoria: 1-40
Von Bonde, C. (1928) Report no. 5 for the years 1925 - 1927. Fisheries and Marine
Biological Survey, Pretoria
Vaarland, S., Boyer, D., and Gibbons, M. (2002) A preliminary study on the diet of the
pelagic goby, Sufflogobius bibarbatus (von bonde), off the Namibian coast.
Southern African Marine Science Symposium (SAMSS 2002): Currents Coasts
Communities. 1-5 Jul 2002, Swakopmund (Namibia)
Venter, J.D. (1976) Voeding van die Suid-Afrikaanse maasbankerTrachurus trachurus
Linnaeus. M.Sc. thesis, Randse Afrikaans University: 174 pp.
Walter, C.B., and O'Neill, E. (1986) Electrophoresis in the study of diets and digestive
rates of seabirds. Comp Bioch Phys A 84A: 763-765
45
BCLME project LMR/CF/03/08
5.2
Mesopelagics
Ahlstrom, E.H., Moser, H.G., and O'Toole, M.J. (1977) Development and distribution of
larvae and early juveniles of the commercial lanternfish, Lampanyctodes hectoris
(Gunther), off the west coast of southern Africa with a discussion of phylogenetic
relationships of the genus. Bull South Calif Acad Sci 75: 138-152
Andronov, V.N. (1983) Feeding of cape horse mackerel (Trachurus trachurus capensis )
and cape hake (Merluccius capensis ) off Namibia in January 1982. Collect Sci
Pap ICSEAF 10: 1-6
Armstrong, M.J., and Prosch, R.M. (1991) Abundance and distribution of the
mesopelagic fish Maurolicus muelleri in the southern Benguela system. S Afr J
Mar Sci 10: 13-28
Assorov, V.V., and Kalinina, M.I. (1979) Some peculiarities of the feeding habits of cape
hake and South African deep-water hake. Collect Sci Pap ICSEAF 6: 219-227
Augustyn, C.J., and Hulley, P.A. (1988) In search of alternative deep-water resources. S
Afr Shipp News Fish Ind Rev 43: 57-59
Castle, P.H.J. (1991) Family Nemichthyidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: 193-194
Centurier-Harris, O.M. (1974) The appearance of lanternfish in commercial catches. S
Afr Shipp News Fish Ind Rev 29: 45
Cohen, D.M. (1991a) Family Argentinidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 215-216
Cohen, D.M. (1991b) Family Bathylagidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 216
Crawford, R.J.M. (1980) Occurrence and distribution of lanternfish Lampancyctodes
hectoris catches in the South African purse-seine fishery, 1968 - 1976. Fish Bull
(S Afr) 13: 111-136
Crawford, R.J.M., Shannon, L.V., and Pollock, D.E. (1987) The Benguela ecosystem.
Part 4. The major fish and invertebrate resources. Aberdeen University Press,
Aberdeen
Cruickshank, R.A. (1982) Possible utilization of lanternfish and gobies in SWA waters. S
Afr Shipp News Fish Ind Rev 37: 29-33
David, J.H.M. (1987) Diet of the South African fur seal (1974-1985) and an assessment
of competition with fisheries in southern Africa. S Afr J Mar Sci 5: 693-713
46
BCLME project LMR/CF/03/08
Ebert, D.A., Compagno, L.J.V., and Cowley, P.D. (1992) A preliminary investigation of
the feeding ecology of squaloid sharks off the west coast of southern Africa. S Afr
J Mar Sci 12: 601-609
Florence, W.K., Hulley, P.A., Stewart, B.A., and Gibbons, M.J. (2002) Genetic and
morphological variation of the lanternfish Lampanyctodes hectoris
(Myctophiformes: Myctophidae) off southern Africa. S Afr J Mar Sci 24: 193-203
Froese, R., and Pauly, D. (2005) FishBase Version 11/2005. World Wide Web electronic
publishing
Garrison, T. (1999) Oceanography: an invitation to marine science. Wadsworth
Publishing, Belmont
Gartner, J.V., Jr. (1991) Life histories of three species of lanternfishes (Pisces:
Myctophidae) from the eastern Gulf of Mexico. 1. Morphological and
microstructural analysis of sagittal otoliths. Mar Biol 111: 11-20
Gartner, J.V., Jr. (1993) Patterns of reproduction in the dominant lanternfish species
(Pisces: Myctophidae) of the eastern Gulf of Mexico, with a review of
reproduction among tropical-subtropical Myctophidae. Bull Mar Sci 52: 721-750
Gibbs, R.H. (1991a) Family Stomiidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 229-230
Gibbs, R.H. (1991b) Family Chauliodontidae. In: Smith, M.M., and Heemstra, P.C.
(Eds). Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 230
Gibbs, R.H. (1991c) Family Astronethidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 231-234
Gibbs, R.H. (1991d) Family Idiacanthidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 234-235
Gibbs, R.H. (1991e) Family Melanostomiidae. In: Smith, M.M., and Heemstra, P.C.
(Eds). Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 236-243
Gjosaeter, J., and Kawaguchi, K. (1980) A review of the world resources of mesopelagic
fish. Fisheries Technical Paper 193. FAO, Rome: 151 p.
Gon, O. (1990) Bathylagidae. In: Gon, O., and Heemstra, P.C. (Eds). Fishes of the
Southern Ocean. J.L.B. Smith Institute of Ichthyology, Grahamstown: pp 107-110
47
BCLME project LMR/CF/03/08
Goodyear, R.H., and Gibbs, R.H. (1991) Family Malacosteidae. In: Smith, M.M., and
Heemstra, P.C. (Eds). Smith's Sea Fishes. Southern Book Publishers,
Johannesburg: pp 235-236
Greely, T.M., Gartner, J.V., Jr., and Torres, J.J. (1999) Age and growth of Electrona
antarctica (Pisces: Myctophidae), the dominant mesopelagic fish of the southern
Ocean. Mar Biol 133: 145
Hewitson, J.D., and Cruickshank, R.A. (1993) Production and consumption by
planktivorous fish in the northern Benguela ecosystem in the 1980s. S Afr J Mar
Sci 13: 15-24
Hulley, P.A. (1981) Results of the Research Cruises of FRV Walther Herwig to South
America. 58. Family Myctophidae (Osteichthyes, Myctophifores). Arch
Fischereiwiss 31: 1-300
Hulley, P.A. (1984) The South African Museum's Meiring naude cruises. Part 14. Family
Myctophidae (Osteichthyes, Myctophiformes). Ann S Afr Mus 93 53-96
Hulley, P.A. (1986) Lanternfishes of the southern Benguela region. Part 1. Faunal
complexity and general distribution. Ann S Afr Mus 97: 227-249
Hulley, P.A., and Prosch, R.M. (1987) Mesopelagic fish derivatives in the southern
Benguela upwelling region. S Afr J Mar Sci 5: 597-611
Hulley, P.A., and Lutjeharms, J.R.E. (1989) Lanternfishes of the southern Benguela
Region. Part 3. The pseudoceanic-oceanic interface. Ann S Afr Mus 98: 409-435
Hulley, P.A. (1990) Family Myctophidae. In: Gon, O., and Heemstra, P.C. (Eds). Fishes
of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown: 146-
178
Hulley, P.A. (1991) Order Myctophiformes. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 282-322
Hulley, P.A. (1992) Upper-slope distributions of oceanic lanternfishes (family:
Myctophidae). Mar Biol 114: 365-383
Huse, I., Hamukuaya, H., Boyer, D.C., Malan, P.E., and Stroemme, T. (1998) The diurnal
vertical dynamics of cape hake and their potential prey. S Afr J Mar Sci 19: 365-
376
Jackson, C.S. (1988) Diets of the white-chinned petrel and sooty shearwater in the
southern Benguela region, South Africa. Condor 90: 20-28
48
BCLME project LMR/CF/03/08
Jackson, G.D., McKinnon, J.F., Lalas, C., Ardern, R., and Buxton, N.G. (1998) Food
spectrum of the deepwater squid Moroteuthis ingens (Cephalopoda:
Onychoteuthidae) in New Zealand waters. Polar Biol 20: 56-65
John, H.C., and Kloppmann, M. (1993) The vertical distribution of eggs of Maurolicus
muelleri. S Afr J Mar Sci 13: 161-174
Johnson, R.K. (1991a) Family Evermannellidae. In: Smith, M.M., and Heemstra, P.C.
(Eds). Smith's Sea Fishes. Southern Book Publishers, Johannesburg: 278-280
Johnson, R.K. (1991b) Family Scopelarchidae. In: Smith, M.M., and Heemstra, P.C.
(Eds). Smith's Sea Fishes. Southern Book Publishers, Johannesburg: 265-268
Konchina, Y.V. (1986) Distribution and feeding of South African horse mackerel and
hake in the Namibian shelf waters. Collect Sci Pap ICSEAF 13: 7-18
Krefft, G. (1991) Family Notosudidae. In: Smith, M.M., and Heemstra, P.C. (Eds).
Smith's Sea Fishes. Southern Book Publishers, Johannesburg: pp 268-270
Matsui, T., and Rosenblatt, R.H. (1991) Family Platytroctidae. In: Smith, M.M., and
Heemstra, P.C. (Eds). Smith's Sea Fishes. Southern Book Publishers,
Johannesburg: 223-225
May, J.L., and Blaber, S.J.M. (1989) Benthic and pelagic fish biomass of the upper
continental slope off eastern Tasmania. Mar Biol 101: 11-25
McClatchie, S., and Dunford, A. (2003) Estimated biomass of vertically migrating
mesopelagic fish off New Zealand. Deep-Sea Res Pt I 50: 1263-1281
Moku, M., Kawaguchi, K., Watanabe, H., and Ohno, A. (2000) Feeding habits of three
dominant myctophid fishes, Diaphus theta, Stenobrachius leucopsarus and S.
nannochir, in the subarctic and transitional waters of the western North Pacific.
Mar Ecol Prog Ser 207: 129-140
Nelson, J.S. (1994) Fishes of the world. John Wiley & Sons, Inc., New York
Nepgen, C.S.d.V. (1979) The food of the snoek Thyrsites atun. Fish Bull (S Afr) 11: 39-
42
Olivar, M.P. (1981) Distribution and abundance of ichthyoplankton caught during the
cruise Benguela 1: November 1979. Collect Sci Pap ICSEAF 8: 161-173
Olivar, M.P., and Rubies, P. (1985) Ichthyoplankton from the southern coast of Namibia
(Walvis Bay-Orange River) during the Benguela V Cruise, July 1983. Collect Sci
Pap ICSEAF 12: 85-97
49
BCLME project LMR/CF/03/08
Olivar, M.P., and Rubies, P. (1986) Early life history of Symbolophorus boops
(Osteichthyes, Myctophidae) in the southeastern Atlantic. Sci Mar 50: 437-447
Olivar, M.P. (1987) Larval development and spawning of Diaphus hudsoni in the
Benguela Current region. Mar Biol 94: 605-611
Olivar, M.P. (1988) Comparative aspects of the ichthyoplankton of the coast of Namibia
and the coast of South Africa in July 1984. Collect Sci Pap ICSEAF 15: 87-126
Olivar, M.P. (1990) Spatial patterns of ichthyoplankton distribution in relation to
hydrographic features in the Northern Benguela region. Mar Biol 106: 39-48
Olivar, M.P., Rubies, P., and Salat, J. (1992) Horizontal and vertical distribution patterns
of ichthyoplankton under intense upwelling regimes off Namibia. S Afr J Mar Sci
12: 71-82
Olivar, M.P., and Shelton, P.A. (1993) Larval fish assemblages of the Benguela current.
Bull Mar Sci 53: 450-474
Olivar, M.P., and Beckley, L.E. (1994) Investigations on the occurrence of larvae of
Symbolophorus species (Myctophidae) off southern Africa. S Afr J Mar Sci 14:
349-359
Olivar, M.P., Salat, J., and Beckley, L.E. (1998) Evidence of displacement of lanternfish
larvae associated with surface water movement: Case studies from southern
Africa. S Afr J Mar Sci 19: 233-244
O'Toole, M.J. (1976) Incidental collections of small and juvenile fishes from egg and
larval surveys off South West Africa (1972-1974). Fish Bull (S Afr): 23-33
O'Toole, M.J. (1977 ) Investigations into some important fish larvae in the southeast
Atlantic in relation to the hydrological environment. Doctor of Philosophy thesis,
University of Cape Town: 308 pp.
Oven, L.S., Konstantinova, M.P., and Shevchenko, N.F. (1990) Aspects of reproduction
and feeding of myctophids (Myctophidae) in the southwest Atlantic. J Ichthyol
30: 115-127
Payne, A.I.L., Rose, B., and Leslie, R.W. (1987) Feeding of hake and a first attempt at
determining their trophic role in the South African west coast marine
environment. S Afr J Mar Sci 5: 471-501
Pillar, S.C., and Barange, M. (1997) Diel variability in bottom trawl catches and feeding
activity of the cape hakes off the west coast of South Africa. ICES J Mar Sci 54:
485-499
50
BCLME project LMR/CF/03/08
Prosch, R.M. (1986) The biology, distribution and ecology of Lampanyctodes hectoris
and Maurolicus muelleri along the South African coast. M.Sc. thesis, University
of Cape Town: 197 pp.
Prosch, R.M. (1991) Reproductive biology and spawning of the myctophid
Lampanyctodes hectoris and the sternoptychid Maurolicus muelleri in the
southern Benguela ecosystem. S Afr J Mar Sci 10: 241-252
Prosch, R.M., Hulley, P.A., and Cruickshank, R.A. (1995) Mesopelagic fish and some
other forage species. In: Payne, A.I.L., and Crawford, R.J.M. (Eds). Ocenas of life
off Southern Africa. Vlaeberg, Cape Town: 130-135
Punt, A.E., Leslie, R.W., and du Plessis, S.E. (1992) Estimation of the annual
consumption of food by Cape hake Merluccius capensis and M. paradoxus off the
South African west coast. S Afr J Mar Sci 12: 611-634
Pusch, C., Fock, H., Porteiro, F., and von Westernhagen, H. (2002) Interaction of
mesopelagic fish and shallow topography in different latitudes of the NE Atlantic.
ICES Council Meeting Documents 2002/M:10. International Council for the
Exploration of the Sea, Copenhagen: 19 p.
Pusch, C., Hulley, P.A., and Kock, K.H. (2004) Community structure and feeding
ecology of mesopelagic fishes in the slope waters of King George Island (South
Shetland Islands, Antarctica). Deep-Sea Res Pt I 51: 1685-1708
Rubiés, P., Bas, C., and Margalef, R. (1985) Zoography of the lanternfishes
(Osteichthyes, Myctophidae) of southwest Africa. In: Bas, C., Margalef, R., and
Rubies, P. (Eds). International symposium on the most important upwelling areas
of Western Africa (Cape Blanco and Benguela). Instituto de Investigaciones
Pesqueras, Barcelona: 573-586
Sabates, A., and Olivar, M.P. (1989) Comparative spawning strategies of mesopelagic
fishes in two marine systems with different productivity. 3rd ICES Symposium on
the Early Life History of Fish. I C E S Marine Science Symposia: 191
Sabates, A., Bozzano, A., and Vallvey, I. (2003) Feeding pattern and the visual light
environment in myctophid fish larvae. J Fish Biol 63: 1476-1490
Salvanes, A.G.V., and Kristoffersen, J.B. (2001) Mesopelagic fishes. In: Steele, J.H.,
Turekian, K.K., and Thorpe, S.A. (Eds). Encyclopedia of Ocean Sciences.
Academic Press, London: 1711-1717
Salvanes, A.G.V. (2004) Mesopelagic fish. In: Skjoldal, H.R., Saetre, R., Faernoe, A.,
Misund, O.A., and Roettingen, I. (Eds). The Norwegian Sea Ecosystem. Tapir
Academic Press, Trondheim: 301-314
51
BCLME project LMR/CF/03/08
Schaefer, S., Johnson, R.K., and Badcock, J. (1991) Family Gonostomatidae. In: Smith,
M.M., and Heemstra, P.C. (Eds). Smith's Sea Fishes. Southern Book Publishers,
Johannesburg: pp 247-253
Schaefer, S., Johnson, R.K., and Badcock, J. (1991) Family Phosichthyidae. In: Smith,
M.M., and Heemstra, P.C. (Eds). Smith's Sea Fishes. Southern Book Publishers,
Johannesburg: pp 243-247
Shannon, L.V. (1985) The Benguela ecosystem. Part I. Evolution of the Benguela,
physical features and processes. Oceanography and Marine Biology Annual
Review 23: 105-182
Shannon, L.V. (1986) Some distinguishing features of the Benguela system. Collect Sci
Pap ICSEAF 13: 225-228
Shannon, L.J., and Jarre-Teichmann, A. (1999) A Model of trophic flows in the northern
Benguela upwelling system during the 1980s. S Afr J Mar Sci 21: 349-366
Shelton, P.A., and Davies, S.L. (1979) Occurrence of lightfish off the Cape coast. S Afr
Shipp News Fish Ind Rev 34: 28-29
Smith, M.M., and Heemstra, P.C. (1991) Smith's Sea Fishes. Southern Book Publishers,
Johannesburg
Strømme, T. (1992) NAN-SIS: Software for fishery survey data logging and analysis.
User's manual. Computerized Information Series. FAO, Rome: 103 p.
Sumaila, U.R., Ninnes, C., and Oelofsen, B. (2003) Management of shared stocks in the
Benguela marine ecosystem. Fisheries Report 695. FAO, Rome: 143-158 p.
Traut, J. (1996) Diet and annual consumption for the cape hakes on the Namibian shelf,
with special reference to cannibalsim. M.Phil. thesis, University of Bergen: 66 pp.
Villanueva, R. (1993) Diet and mandibular growth of Octopus magnificus Cephalopoda).
S Afr J Mar Sci 13: 121-126
Watanabe, H., Moku, M., Kawaguchi, K., Ishimaru, K., and Ohno, A. (1999) Diel
vertical migration of myctophid fishes (Family Myctophidae) in the transitional
waters of the western North Pacific. Fish Oceanogr 8: 115-127
Watanabe, H., Kawaguchi, K., and Hayashi, A. (2002) Feeding habits of juvenile surface-
migratory myctophid fishes (family Myctophidae) in the Kuroshio region of the
western North Pacific. Mar Ecol Prog Ser 236: 263-272
Weitzman, S.H. 1991. Family Sternoptychidae. In: Smith, M.M., and Heemstra, P.C.
(Eds). Smith's Sea Fishes. Southern Book Publishers, Johannesburg: 253-259
52
BCLME project LMR/CF/03/08
Williams, A., and Koslow, J.A. (1997) Species composition, biomass and vertical
distribution of micronekton over the mid-slope region off southern Tasmania,
Australia. Mar Biol 130: 259-276
Williams, A., Koslow, J.A., Terauds, A., and Haskard, K. (2001) Feeding ecology of five
fishes from the mid-slope micronekton community off southern Tasmania,
Australia. Mar Biol 139: 1177-1192
Young, J.W., and Blaber, S.J.M. (1986) Feeding ecology of three species of midwater
fishes associated with the continental slope of eastern Tasmania, Australia. Mar
Biol 93: 147-156
Young, J.W., Blaber, S.J.M., and Rose, R. (1987) Reproductive biology of three species
of midwater fishes associated with the continental slope of Eastern Tasmania,
Australia. Mar Biol 95: 323-332
Young, J.W., Bulman, C.M., Blader, S.J.M., and Wayte, S.E. (1988) Age and growth of
the lanternfish Lampanyctodes hectoris (Myctophidae) from eastern Tasmania,
Australia. Mar Biol 99: 569-576
53
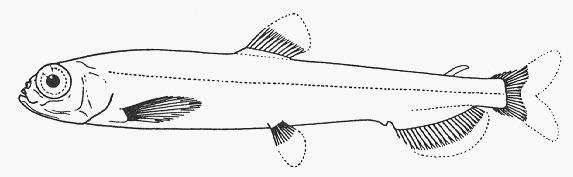
BCLME project LMR/CF/03/08
Annex 1: Picture gallery of some mesopelagic species
Order Perciformes
Order Anguilliformes
Family Gobiidae
Family Nemichthyidae (snipe eels)
Species Sufflogobius bibarbatus
Species Nemichthys scolopaceus
Order Argentiformes
Family Bathylagidae (deep-sea smelts)
Species Bathylagus antarcticus
Species Bathylagus bericoides
Order Stomiiformes
Family Gonostomatidae (bristlemouths)
Species Gonostoma denudatum
Species Diplophos maderensis
Family Sternoptychidae (marine hatchetfishes)
Species Argyropelecus aculeatus
Species Maurolicus muelleri
NOTE: All pictures were obtained from Fish Base (2005). P.C. Heemstra from the South African Institute for Biodiversity provided
the originals to FishBase.
54
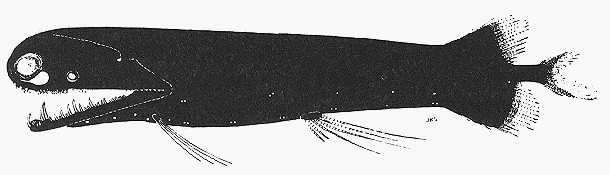
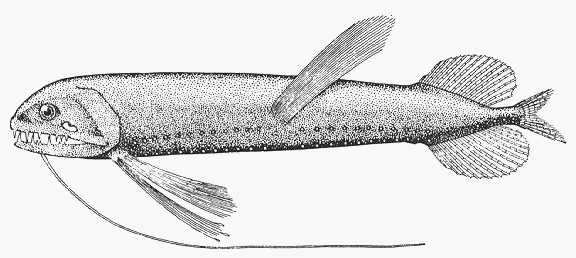
BCLME project LMR/CF/03/08
Annex 2: Picture gallery of some mesopelagic species
Order Stomiiformes
Family Photichthyidae (lightfishes)
Species Yarella blackfordi
Species Photichthys argenteus
Family Stomiidae (scaly dragonfishes)
Family Chauliodontidae (viperfishes)
Species Stomias boa boa
Species Chauliodus sloani
Family Astronesthidae (snaggletooths)
Family Idiacanthidae (sawtail fish)
Species Borostomias monomena
Species Idiacanthus atlanticus
Family Malacosteidae (loosejaws)
Family Melanostomiatidae
Species Malacosteus niger
Species Bathophilus nigerrimus
NOTE: All pictures were obtained from Fish Base (2005). P.C. Heemstra from the South African Institute for Biodiversity provided
the originals to FishBase.
55
BCLME project LMR/CF/03/08
Annex 3: Picture gallery of some mesopelagic species
Order Aulopiformes
Family Notosudidae (waryfishes)
Family Evermannellidae
Species Scopelosaurus meadi
Species Evermannella balbo
Family Scopelarchidae (pearleyes)
Species Benthalbella macropinna
Order Myctophiformes
Family Myctophidae (lanternfishes)
Family Neoscopelidae (blackchins)
Species Lampanyctodes hectoris
Species Neoscopelus macrolepidotus
NOTE: All pictures were obtained from Fish Base (2005). P.C. Heemstra from the South African Institute for Biodiversity provided
the originals to FishBase.
56
BCLME project LMR/CF/03/08
Annex 4: Nan-Sis database mesopelagic species listed - Angola
Table A: Families and species captured in the Nan-Sis species catalogue AN, searched for and
found in Angolan projects AN, A2, A3 and A4 (Families in alphabetic order).
# of stations *
Family
Genus / Species
Database code
AN
A2
A3
A4
Argentinidae
ARGAA00
---
---
---
2
Astronesthidae
ASTAA00
---
---
2
12
Astronesthes sp.
ASTAS00
---
---
---
1
Borostomias sp.
ASTBO00
---
---
1
3
Bathylagidae
BAHAA00
---
---
---
---
Bathylagus sp.
BAHBA00
---
---
---
2
Bathylagus longirostris
BAHBA01
---
---
1
---
Bathylagus bericoides
BAHBA02
---
1
---
---
Bathylagus glacilis
BAHBA03
---
---
---
3
Chauliodontidae
CHOAA00
---
---
---
3
Chauliodus sp.
CHOCH00
---
---
---
3
Chauliodus sloani
CHOCH01
---
---
---
18
Gobiidae
GOBAA00
27
38
10
134
Gobies sp.
GOBSG00
---
---
---
---
Sufflogobius bibarbatus
GOBSU01
---
---
---
33
Thorogobius angolensis
GOBTH01
---
---
11
20
Gonostomatidae
GONAA00
6
8
55
156
Diplophos sp.
GONDI00
---
1
---
19
Gonostoma sp.
GONGO00
---
---
---
41
Gonostoma elongatum
GONGO01
---
3
---
45
Gonostoma denudata
GONGO02
---
---
---
43
Triplophos sp.
GONTR00
---
5
4
111
Triplophos hemingi
GONTR01
---
---
---
197
Malacosteidae
MAAAA00
---
---
---
---
Melanostomatidae
MELAA00
---
7
30
119
Leptostomias gracilis
MELLE01
---
---
1
---
Melanostomias sp.
MELME00
---
6
3
56
Melanostomias
macrophotus
MELME01
---
---
---
1
Photonectes braueri
MELPH01
---
---
2
19
Photonectes parvimanus
MELPH02
---
---
---
---
Myctophidae
MYCAA00
64
36
60
703
Diaphus sp.
MYCDI00
---
---
---
17
Diaphus dumerili
MYCDI03
---
1
40
5
Lampadena sp.
MYCLA00
---
1
--
7
Myctophum sp.
MYCMY00
---
---
1
7
Notoscopelus sp.
MYCNO00
1
---
---
---
Nemichthyidae
NECAA00
---
2
3
32
Avocettina acuticeps
NECAV01
---
---
---
---
Nemichthys sp.
NECNE00
---
---
---
---
Nemichthys scolopaceus
NECNE01
---
7
7
144
Nemichthys curvirostris
NECNE02
---
---
---
4
Notosudidae
NOSAA00
---
---
---
6
Scopelosaurus sp.
NOSSC00
---
---
---
14
Scopelosaurus meadi
NOSSC01
---
---
---
1
Photichthyidae
PHOAA00
---
---
---
---
57
BCLME project LMR/CF/03/08
Annex 4: Nan-Sis database mesopelagic species listed Angola contnd.
Table B: Families and species captured in the Nan-Sis species catalogue AN, searched for and
found in Angolan projects AN, A2, A3 and A4 (Families in alphabetic order).
# of stations *
Family
Genus / Species
Database code
AN
A2
A3
A4
Photichthys sp.
PHOPH00
---
---
---
---
Yarella blackfordi
PHOYA02
---
23
6
387
Platytroctidae
PLTAA00
---
---
---
8
Sternoptychidae
STEAA00
---
---
---
4
Argyropelecus affinis
STEAR02
---
---
---
2
Maurolicus muelleri
STEMA01
---
---
4
51
Sternoptyx sp.
STEST00
---
1
---
1
Sternoptyx pseudobscura
STEST01
---
---
---
---
Stomiidae
STOAA00
2
2
1
72
Stomias sp.
STOST00
---
---
---
61
Stomias affinis
STOST01
---
3
9
28
Stomias boa boa
STOST02
---
---
---
125
* Stations searched: AN 1-992 ; A2 1-531; A3 1-475; A4 1-3896
58
BCLME project LMR/CF/03/08
Annex 5: Nan-Sis database mesopelagic species listed - Namibia
Table A: Families and species captured in the Nan-Sis species catalogue NA, searched for and
found in Namibian projects NA, N1, N2 and NC (Families in alphabetic order).
# of stations *
Family
Genus / Species
Database code
NA
N1
N2
NC
Argentinidae
ARGAA00
---
1
---
---
Nansenia problematica
ARGNA02
1
1
---
---
Astronesthidae
ASTAA00
2
8
---
5
Bathylagidae
BAHAA00
---
10
---
5
Bathylagus glacilis
BAHBA03
2
29
18
76
Nansenia sp.
BAHNA00
3
2
---
---
Nansenia problematica
BAHNA02
1
1
---
2
Nansenia tenera
BAHNA03
1
1
---
---
Chauliodontidae
CHOAA00
---
2
---
1
Chauliodus sloani
CHOCH01
---
7
---
6
Gobiidae
GOBAA00
---
3
---
1
Sufflogobius bibarbatus
GOBSU01
601
630
104
529
Gonostomatidae
GONAA00
20
54
9
225
Diplophos sp.
GONDI00
4
1
---
2
Diplophos maderensis
GONDI02
7
1
---
---
Triplophos sp.
GONTR00
---
16
9
6
Idiacanthidae
IDIAA00
1
---
---
---
Idiacanthus atlanticus
IDIID01
2
---
---
---
Malacosteidae
MAAAA00
---
4
2
24
Melanostomatidae
MELAA00
4
5
10
38
Leptostomias gracilis
MELLE01
---
2
---
---
Melanostomias sp.
MELME00
---
9
---
---
Odontostomias
micropogon
MELOD01
1
2
---
---
Photonectes braueri
MELPH01
6
2
---
1
Myctophidae
MYCAA00
429
568
26
142
Diaphus sp.
MYCDI00
11
25
1
7
Diaphus dumerili
MYCDI03
---
16
---
---
Diaphus hudsoni
MYCDI05
---
8
5
22
Lampadena sp.
MYCLA00
5
8
---
5
Lampanyctodes hectoris
MYCLM01
28
98
35
125
Symbolophorus boobs
MYCSY01
---
75
38
143
Symbolophorus barnardi
MYCSY02
---
---
---
---
Nemichthyidae
NECAA00
6
31
4
10
Nemichthys scolopaceus
NECNE01
27
30
8
70
Nemichthys curvirostris
NECNE02
4
6
---
---
Neoscopelidae
NEOAA00
---
2
----
---
Neoscopelus
macrolepidotus
NEONE01
3
31
2
24
Neoscopelus microchir
NEONE02
2
---
---
1
Notosudidae
NOSAA00
---
9
---
9
Scopelosaurus meadi
NOSSC01
3
27
1
5
Photichthyidae
PHOAA00
3
15
1
6
Photichthys sp.
PHOPH00
---
14
1
14
Photichthys argenteus
PHOPH01
55
143
19
79
Yarella blackfordi
PHOYA02
46
325
49
264
59
BCLME project LMR/CF/03/08
Annex 5: Nan-Sis database mesopelagic species listed Namibia contnd.
Table B: Families and species captured in the Nan-Sis species catalogue NA, searched for and
found in Namibian projects NA, N1, N2 and NC (Families in alphabetic order).
# of stations *
Family
Genus / Species
Database code
NA
N1
N2
NC
Sternoptychidae
STEAA00
1
1
---
1
Argyropelecus affinis
STEAR02
2
4
---
3
Maurolicus muelleri
STEMA01
37
93
14
25
Stomiidae
STOAA00
---
22
---
16
Stomias boa boa
STOST02
4
31
16
105
* Stations searched: N1 1-2600; N2 2596-2980; NA 1-2090; NC 2231-4070
60
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa
Table A: Number of stations of families and species captured in the Nan-Sis species catalogue
SA (Families in alphabetic order).
Family
Genus / Species
Database code # of stations
Argentinidae
ARGAA00
---
Argentina euchus
ARGAR05
9
Astronesthidae
ASTAA00
1
Astronesthes sp.
ASTAS00
7
Astronesthes filifer
ASTAS06
---
Borostomias sp.
ASTBO00
1
Borostomias monomena
ASTBO02
1
Borostomias antarcticus
ASTBO03
3
Neonesthes capensis
ASTNE01
6
Bathylagidae
BAHAA00
---
Bathylagus sp.
BAHBA00
10
Bathylagus glacilis
BAHBA03
---
Bathylagus antarcticus
BAHBA04
3
Nansenia sp.
BAHNA00
2
Nansenia problematica
BAHNA02
---
Nansenia tenera
BAHNA03
---
Chauliodontidae
CHOAA00
1
Chauliodus sloani
CHOCH01
35
Evermannellidae
EVEAA00
2
Evermannella balbo
EVEEV01
---
Gobiidae
GOBAA00
---
Gobidae juvenile
GOBAA90
---
Sufflogobius bibarbatus
GOBSU01
98
Gonostomatidae
GONAA00
10
Diplophos sp.
GONDI00
---
Diplophos taenia
GONDI01
5
Diplophos maderensis
GONDI02
---
Gonostoma elongatum
GONGO01
11
Cyclothone sp.
GONSY00
4
Triplophos sp.
GONTR00
---
Triplophos hemingi
GONTR01
---
Idiacanthidae
IDIAA00
1
Idiacanthus sp.
IDIID00
1
Idiacanthus atlanticus
IDIID01
9
Malacosteidae
MAAAA00
---
Aristostomias sp.
MAAAR00
2
Malacosteus sp.
MAAMA00
3
Malacosteus niger
MAAMA01
26
Melanostomatidae
MELAA00
8
Bathophilus longipinnis
MELBA02
23
Bathophilus nigerrimus
MELBA03
1
Echiostoma barbatum
MELEC01
9
Eustomias sp.
MELAU00
---
Leptostomias gracilis
MELLE01
---
Melanostomias sp.
MELME00
8
Odontostomias
micropogon
MELOD01
---
61
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table B: Number of stations of families and species captured in the Nan-Sis species catalogue
SA (Families in alphabetic order).
Family
Genus / Species
Database code # of stations
Opostomias micripnis
MELOP01
---
Photonectes braueri
MELPH01
2
Myctophidae
MYCAA00
73
Bolanichthys
supralateralis
MYCBO01
2
Diaphus sp.
MYCDI00
104
Diaphus dumerili
MYCDI03
---
Diaphus hudsoni
MYCDI05
---
Diaphus effulgens
MYCDI07
23
Diaphus meadi
MYCDI08
4
Diogenichthys panurgus
MYCDO01
1
Electrona risso
MYCEL01
51
Gymnoscopelus sp.
MYCGY00
66
Gymnoscopelus bolini
MYCGY01
2
Hygophum sp.
MYCHY00
1
Lampadena sp.
MYCLA00
14
Lampadena luminosa
MYCLA01
2
Lampadena pontifex
MYCLA04
1
Lampichthys procerus
MYCLC01
3
Lampanyctodes hectoris
MYCLM01
338
Lampanyctus sp.
MYCLP00
9
Lampanyctus alatus
MYCLP02
1
Lampanyctus australis
MYCLP10
3
Myctophum sp.
MYCMY00
15
Notoscopelus sp.
MYCNO00
4
Protomyctophum sp.
MYCPR00
6
Scopelopsis
multipunctatus
MYCSC01
1
Symbolophorus sp.
MYCSY00
21
Symbolophorus boops
MYCSY01
156
Symbolophorus barnardi
MYCSY02
11
Nemichthyidae
NECAA00
16
Avocettina sp.
NECAV00
1
Avocettina acuticeps
NECAV01
16
Nemichthys scolopaceus
NECNE01
13
Nemichthys curvirostris
NECNE02
24
Neoscopelidae
NEOAA00
---
Neoscopelus
macrolepidotus
NEONE01
28
Neoscopelus microchir
NEONE02
---
Notosudidae
NOSAA00
17
Luciosudis normanni
NOSLU01
1
Scopelosaurus sp.
NOSSC00
14
Scopelosaurus meadi
NOSSC01
23
Scopelosaurus herwigi
NOSSC02
10
Photichthyidae
PHOAA00
3
Photichthys sp.
PHOPH00
---
62
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table C: Number of stations of families and species captured in the Nan-Sis species catalogue
SA and the Africana database (Families in alphabetic order).
Family
Genus / Species
Database code # of stations
Photichthyidae
Photichthys argenteus
PHOPH01
251
Yarella blackfordi
PHOYA02
1
Platytroctidae
PLTAA00
4
Scopelarchidae
SCPAA00
5
Benthalbella macropinna
SCPBE02
4
Sternoptychidae
STEAA00
---
Argyropelecus sp.
STEAR00
20
Argyropelecus aculeatus
STEAR01
11
Argyropelecus affinis
STEAR02
---
Maurolicus muelleri
STEMA01
269
Polyipnus
Sternoptyx sp.
STEST00
---
Sternoptyx diaphana
STEST02
14
Argyropelecus gigas
STEST04
3
Stomiidae
STOAA00
3
Opostomias micripnis
Stomias sp.
STOST00
---
Stomias boa boa
STOST02
5
* Stations searched: SA 1-1151
63
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table D: Number of stations for| families and genera / species captured in the Africana
Demersal Data System (Families in alphabetic order).
Family/Sub Family
Genus / Species
Species code # of stations
Argentinidae
Argentina euchus
109K
2
Glossanodon
Astronesthidae
Astronesthes
103M
9
Astronesthes boulengeri
104N
Astronesthes indicus
104M
Astronesthes niger
104G
Borostomias antarcticus
104U
1
Borostomias mononema
103Y
1
Neonesthes capensis
103X
6
Neonesthes microcephalus
103W
1
Bathylagidae
Bathylagus
110G
12
Bathylagus antarcticus
110H
7
Chauliodontidae
104K
14
Chauliodus sloani
104L
50
Evermannellidae
119P
2
Coccorella atlantica
124G
Gobiidae
Gobiidae
Caffrogobius caffer
Caffrogobius nudiceps
Caffrogobius saldanha
Psammogobius knysnaensis
Speckled Goby
Sufflogobius bibarbatus
335
313
Gonostomatidae
105A
14
Cyclothone
103Z
5
Diplophos
105P
Diplophos taenia
105N
3
Gonostoma elongatum
105B
7
Idiacanthidae
Idiacanthus
104P
6
Idiacanthus atlanticus
600
16
Idiacanthus niger
104Q
Malacosteidae
Aristostomias
105G
Malacosteus
104R
7
Malacosteus niger
104S
33
Melanostomiidae
105E
15
Bathophilus ater
108B
Bathophilus nigerrimus
108K
Echiostoma barbartum
105F, 109A
18
Eustomias
109B
4
Eustomias bulbornatus
109C
Eustomias filifer
104W
Eustomias grandibulbus
104V
Eustomias lipochirus
105L
64
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table E: Number of stations of families and genera / species captured in the Africana Demersal
Data System (Families in alphabetic order).
Family / Sub Family Genus / Species
Species code # of stations
Melanostomiidae
Eustomias schmitdti
105J
Melanostomias
105I
5
Melanostomias niger
105Q
3
Melanostomias valdiviae
105R
Opostomias micripnis
105D
1
Photonectes
105M
2
Photonectes braueri
105H
Myctophidae
120A
92
Benthosema suborbitale
122G
Bolinichthys supralateralis
122
4
Ceratoscopelus warmingii
121E
Diaphus
120D
85
Diaphus diadematus
120Q
Diaphus effulgens
119Y
1
Diaphus hudsoni
120H
18
Diaphus lucidus
120N
1
Diaphus meadi
119Z
4
Diaphus mollis
120Z
Diaphus ostenfeldi
120I
2
Diaphus persipicillatus
119V
Diaphus problematicus
120L
Diogenichthys panurgus
120DP
1
Electrona risso
120K
23
Gymnoscopelus
121K
20
Gymnoscopelus bolini
121
2
Gymnoscopelus piabilis
121A
1
Hygophum
121B
3
Hygophum hanseni
121H
Hygophum hygomii
121C
Lampadena
122F
8
Lampadena luminosa
122E
2
Lampadena notialis
122D
Lampadena speculigera
122B
Lampanyctodes hectoris
120B
728
Lampanyctus
120E
22
Lampanyctus achirus
120Y
Lampanyctus alatus
119T
1
Lampanyctus ater
120W
Lampanyctus australis
120T
3
Lampanyctus festivus
120X
Lampanyctus intricarius
120S
Lampanyctus lepidolychnus
120U
Lampanyctus macdonaldi
120V
65
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table F: Number of stations of families and genera / species captured in the Africana Demersal
Data System (Families in alphabetic order).
Family/Sub Family
Genus / Species
Species code # of stations
Myctophidae
Lampanyctus pusillus
119W
1
Lampanyctus tenuiformis
119R
Lampanyctus turneri
119X
Lampichthys procerus
120M
3
Lepidophanes guentheri
120R
Lobianchia
122A
Lobianchia dofleini
121Z
Metelectrona ventralis
120G
2
Myctophum
119L
5
Myctophum nitidulum
119Q
Myctophum phengodes
119U
Myctophum selenops
120P
32
Notoscopelus
121I
1
Notoscopelus caudispinosus
121F
Notoscopelus resplendens
121G
Protomyctophum
121J
8
Protomyctophum andriashevi
121D
Scopelopsis multipunctatus
122C
1
Symbolophorus
120C
24
Symbolophorus barnardi
120J
25
Symbolophorus boops
120F
114
Taaningichthys bathyphilus
119N
Nemichthyidae
460A
33
Avocettina
460D
18
Nemichthys
Nemichthys curvirostris
460C
8
Nemichthys scolopaceus
460B
11
Neoscopelus microchir
Notosudidae
125A
25
Luciosudis normani
125E
1
Scopelosaurus
125
14
Scopelosaurus ahlstromi
125C
3
Scopelosaurus herwigi
125B
2
Scopelosaurus meadi
125D
4
Photichthyidae
104Z
8
Photichthys argenteus
105C
305
Polymetme corythaeola
105S
Platytroctidae
103C
4
Microrictus taaningi
519
Persparsia kopua
103B
6
Sagamichthys schnackenbecki
103A
Scopelarchidae
123
7
Benthalbella elongatum
123B
66
BCLME project LMR/CF/03/08
Annex 6: Database mesopelagic species listed - South Africa contnd.
Table G: Number of stations of families and genera / species captured in the Africana Demersal
Data System (Families in alphabetic order).
Family/Sub Family
Genus / Species
Species code # of stations
Scopelarchidae
Benthalbella macropinna
123A
4
Sternoptychidae
Argyropelecus
107A
27
Argyropelecus aculeatus
107F
25
Argyropelecus gigas
107E
4
Argyropelecus hemigymnus
107B
5
Maurolicus muelleri
106
694
Polyipnus
105T
2
Polyipnus indicus
105U
1
Sternoptyx diaphana
107D
19
Valenciennellus tripunctulatus
106A
Stomiidae
104D
4
Macrostomias longibarbatus
103K
1
Stomias boa
104E
8
67
BCLME project LMR/CF/03/08
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT
Table A: Families and species captured in the Nan-Sis species catalogue AN, NA and SA, and
searched for in the project BE (Families in alphabetic order).
# of stations
Family
Genus / Species
Database code
BE
Argentinidae
ARGAA00
---
Argentina euchus
ARGAR05
---
Astronesthidae
ASTAA00
1
Astronesthes sp.
ASTAS00
---
Astronesthes filifer
ASTAS06
---
Borostomias sp.
ASTBO00
---
Borostomias monomena
ASTBO02
---
Borostomias antarcticus
ASTBO03
---
Neonesthes capensis
ASTNE01
---
Bathylagidae
BAHAA00
---
Bathylagus sp.
BAHBA00
---
Bathylagus longirostris
BAHBA01
---
Bathylagus bericoides
BAHBA02
---
Bathylagus glacilis
BAHBA03
8
Bathylagus antarcticus
BAHBA04
---
Nansenia sp.
BAHNA00
---
Nansenia problematica
BAHNA02
5
Nansenia tenera
BAHNA03
---
Chauliodontidae
CHOAA00
1
Chauliodus sp.
CHOCH00
---
Chauliodus sloani
CHOCH01
2
Evermannellidae
EVEAA00
---
Evermannella balbo
EVEEV01
---
Gobiidae
GOBAA00
15
Gobidae juvenile
GOBAA90
---
Sufflogobius bibarbatus
GOBSU01
93
Thorogobius angolensis
GOBTH01
5
Gonostomatidae
GONAA00
26
Diplophos sp.
GONDI00
---
Diplophos taenia
GONDI01
---
Diplophos maderensis
GONDI02
---
Gonostoma sp.
GONGO00
---
Gonostoma elongatum
GONGO01
---
Gonostoma denudata
GONGO02
---
Cyclothone sp.
GONSY00
---
Triplophos sp.
GONTR00
6
Triplophos hemingi
GONTR01
22
Idiacanthidae
IDIAA00
---
Idiacanthus sp.
IDIID00
---
Idiacanthus atlanticus
IDIID01
---
Malacosteidae
MAAAA00
6
Aristostomias sp.
MAAAR00
---
Malacosteus sp.
MAAMA00
---
Malacosteus niger
MAAMA01
---
Melanostomiatidae
MELAA00
2
Bathophilus longipinnis
MELBA02
---
Bathophilus nigerrimus
MELBA03
---
68
BCLME project LMR/CF/03/08
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT
contnd.
Table B: Families and species captured in the Nan-Sis species catalogue AN, NA and SA, and
searched for in the project BE (families in alphabetic order).
# of stations
Family
Genus / Species
Database code
BE
Melanostomiatidae
Echiostoma barbatum
MELEC01
---
Eustomias sp.
MELAU00
---
Leptostomias gracilis
MELLE01
---
Melanostomias sp.
MELME00
---
Melanostomias
macrophotus
MELME01
---
Odontostomias
micropogon
MELOD01
---
Opostomias micripnis
MELOP01
---
Photonectes braueri
MELPH01
---
Photonectes parvimanus
MELPH02
---
Myctophidae
MYCAA00
178
Bolanichthys
supralateralis
MYCBO01
---
Diaphus sp.
MYCDI00
19
Diaphus dumerili
MYCDI03
---
Diaphus hudsoni
MYCDI05
25
Diaphus effulgens
MYCDI07
---
Diaphus meadi
MYCDI08
---
Diogenichthys panurgus
MYCDO01
---
Electrona risso
MYCEL01
---
Gymnoscopelus sp.
MYCGY00
---
Gymnoscopelus bolini
MYCGY01
---
Hygophum sp.
MYCHY00
---
Lampadena sp.
MYCLA00
11
Lampadena luminosa
MYCLA01
---
Lampadena pontifex
MYCLA04
---
Lampichthys procerus
MYCLC01
---
Lampanyctodes hectoris
MYCLM01
120
Lampanyctus sp.
MYCLP00
---
Lampanyctus alatus
MYCLP02
---
Lampanyctus australis
MYCLP10
---
Myctophum sp.
MYCMY00
---
Notoscopelus sp.
MYCNO00
---
Protomyctophum sp.
MYCPR00
---
Scopelopsis
multipunctatus
MYCSC01
---
Symbolophorus sp.
MYCSY00
---
Symbolophorus boops
MYCSY01
65
Symbolophorus barnardi
MYCSY02
---
Nemichthyidae
NECAA00
1
Avocettina sp.
NECAV00
---
Avocettina acuticeps
NECAV01
---
Nemichthys sp.
NECNE00
---
Nemichthys scolopaceus
NECNE01
8
Nemichthys curvirostris
NECNE02
---
69
BCLME project LMR/CF/03/08
Annex 7: Nan-Sis database mesopelagic species listed BENEFIT
contnd.
Table C: Families and species captured in the Nan-Sis species catalogue AN, NA and SA, and
searched for in the project BE (families in alphabetic order).
# of stations
Family
Genus / Species
Database code
BE
Neoscopelidae
NEOAA00
---
Neoscopelus
macrolepidotus
NEONE01
2
Neoscopelus microchir
NEONE02
---
Notosudidae
NOSAA00
1
Luciosudis normanni
NOSLU01
---
Scopelosaurus sp.
NOSSC00
---
Scopelosaurus meadi
NOSSC01
6
Scopelosaurus herwigi
NOSSC02
---
Photichthyidae
PHOAA00
6
Photichthys sp.
PHOPH00
1
Photichthys argenteus
PHOPH01
30
Yarella blackfordi
PHOYA02
97
Platytroctidae
PLTAA00
---
Scopelarchidae
SCPAA00
---
Benthalbella macropinna
SCPBE02
---
Sternoptychidae
STEAA00
1
Argyropelecus sp.
STEAR00
---
Argyropelecus aculeatus
STEAR01
---
Argyropelecus affinis
STEAR02
2
Maurolicus muelleri
STEMA01
72
Sternoptyx sp.
STEST00
---
Sternoptyx pseudobscura
STEST01
---
Sternoptyx diaphana
STEST02
---
Argyropelecus gigas
STEST04
---
Stomiidae
Stomias sp.
STOST00
4
Stomias affinis
STOST01
---
Stomias boa boa
STOST02
37
* Stations searched: BE 1 - 1650
70
BCLME project LMR/CF/03/08
Annex 8: Distribution maps - Order Anguilliformes and Argentiformes
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
S
Namibia
-25
-25
a
t
i
t
u
d
e
L
-30
-30
South Africa
-35
-35
A
B
-40
-40
10
15
20
25
10
15
20
25
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
Namibia
-25
-25
t
i
t
u
d
e
S
L
a
-30
-30
South Africa
-35
-35
C
D
-40
-40
10
15
20
25
10
15
20
25
Longitude East ('E)
Longitude East ('E)
Figure 1: A) Nemichthyidae, B) Argentinidae, C) Bathylagidae, D) Platytroctidae
71
BCLME project LMR/CF/03/08
Annex 9: Distribution maps - Order Stomiiformes
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
S
Namibia
-25
-25
a
t
i
t
u
d
e
L
-30
-30
South Africa
-35
-35
A
B
-40
-40
10
15
20
25
10
15
20
25
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
Namibia
-25
-25
t
i
t
u
d
e
S
L
a
-30
-30
South Africa
-35
-35
C
D
-40
-40
10
15
20
25
10
15
20
25
Longitude East ('E)
Longitude East ('E)
Figure 1: A) Stomiidae, B) Chauliodontidae, C) Astronesthidae, D) Idiacanthidae
72
BCLME project LMR/CF/03/08
Annex 10: Distribution maps - Order Stomiiformes continued
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
S
Namibia
-25
-25
a
t
i
t
u
d
e
L
-30
-30
South Africa
-35
-35
A
B
-40
-40
10
15
20
25
10
15
20
25
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
Namibia
-25
-25
t
i
t
u
d
e
S
L
a
-30
-30
South Africa
-35
-35
C
D
-40
-40
10
15
20
25
10
15
20
25
Longitude East ('E)
Longitude East ('E)
Figure 1: A) Malacosteidae, B) Photichthyidae, C) Gonostomatidae, D) Sternoptychidae
73
BCLME project LMR/CF/03/08
Annex 11: Distribution maps - Order Stomiiformes and Aulopiformes
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
S
Namibia
-25
-25
a
t
i
t
u
d
e
L
-30
-30
South Africa
-35
-35
A
B
-40
-40
10
15
20
25
10
15
20
25
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
Namibia
-25
-25
t
i
t
u
d
e
S
L
a
-30
-30
South Africa
-35
-35
C
D
-40
-40
10
15
20
25
10
15
20
25
Longitude East ('E)
Longitude East ('E)
Figure 1: A) Melanostomatidae, B) Scopelarchidae, C) Notosudidae, D) Evermannellidae
74
BCLME project LMR/CF/03/08
Annex 12: Distribution maps - Order Myctophiformes
-10
-10
Angola
-15
-15
)
-20
-20
t
h
(
'
S
o
u
Namibia
d
e
S
-25
-25
t
i
t
u
L
a
-30
-30
South Africa
-35
-35
A
B
-40
-40
10
15
20
25
10
15
20
25
Figure 1: A) Myctophidae, B) Neoscopelidae
75
BCLME project LMR/CF/03/08
Annex 13: Distribution of gobies and mesopelagic species collected by
R.V. Welwitchia during horse mackerel surveys.
16°S
Mesopelagics and Gobies
Tiger Bay
Cunene River
18°S
Cape Frio
Mowe Bay
20°S
Ambrose Bay
22°S
Walvis Bay
24°S
Conception Bay
Easter point
26°S
Luderitz
28°S
200 m
500 m
12°E
14°E
16°E
Figure 1: Distribution of catches with gobies (blue) and mesopelagic species (brown) recorded on
the R.V. Welwithcia. More information can be obtained from Angie Kanandjembo, NatMIRC.
76
BCLME project LMR/CF/03/08
Annex 14: Catch history
Table A: Landings (t) of lanternfish and gobiidae in South African and Namibian waters (Source
FishBase 2005).
Year Gobiidae SA Gobiidae NAM Lanternfish SA *
1965
0
0
0
1966
0
0
0
1967
0
0
0
1968
0
0
0
1969
0
0
0
1970
0
0
18,200
1971
0
0
2,600
1972
0
0
15,200
1973
0
0
42,400
1974
0
0
301
1975
0
0
87
1976
0
0
132
1977
55
0
5,650
1978
62
0
950
1979
300
0
9,885
1980
10
0
40
1981
409
0
10,278
1982
35
0
675
1983
1,254
0
1,633
1984
0
0
13,553
1985
3
0
30,993
1986
626
0
313
1987
185
0
22
1988
32
0
144
1989
0
0
4,704
1990
0
0
571
1991
0
0
662
1992
0
86
656
1993
0
172
1,177
1994
0
19
871
1995
0
5
862
1996
0
552
33
1997
0
287
243
1998
0
20,998
6,553
1999
0
16
0
2000
0
3
0
2001
0
0
0
*Lampanyctodes hectoris
77
BCLME project LMR/CF/03/08
Annex 15: BENEFIT survey proposal on mesopelagics
BENEFIT PROJECT PROPOSAL AND APPLICATION FOR FUNDING
January 2006
1.
TITLE OF PROJECT: Acoustic survey of the meso-pelagic resources of the
Benguela region
2.
DURATION OF PROJECT:
January 2006 to December 2007 (2 years)
3.
CATEGORY OF PROJECT:
First proposal
4.
KEYWORDS BY WHICH PROJECT CAN BE IDENTIFIED:
acoustic surveys, mesopelagic fish, Target strength
5.
RESPONSIBLE PROJECT LEADER (AND CO-LEADERS):
Project leaders:
Name
Janet Coetzee
Business address
Marine and Coastal Management
P. Bag X2, Roggebaai, 8012
South Africa
Telephone
(021) 4023174
E-mail
jcoetzee@deat.gov.za
Name
Graca de Almeida
Business address
NatMIRC, P.O.Box 912
Swakopmund
E-mail
gdalmeida@mfmr.gov.na
Collaborators:
Name
Martha Uumati
Business address
NatMIRC, P.O.Box 912
Swakopmund
E-mail
muumati@mfmr.gov.na
Name
Carl van der Lingen
Business address
Marine and Coastal Management
P. Bag X2, Roggebaai, 8012
South Africa
E-mail
Vdlingen@deat.gov.za
78
BCLME project LMR/CF/03/08
Name
Arved Staby
Business address
Universitetet i Bergen
Institutt for biologi
Postboks 7800
N-5020 Bergen
E-mail
arved.staby@bio.uib.no
6.
SUMMARY OF FINANCIAL REQUIREMENTS:
This application 2006 Following year (est.) 2007
Vessel costs
21 days
-
Running expenses
N$ 5 000
N$ 5 000
Travel and per diem
N$ 55 000
N$ 80 000
TOTAL
N$ 60 000
N$ 85 000
7.
ORGANISATION RESPONSIBLE FOR ADMINISTRATION OF THE
FUNDS
BENEFIT Secretariat
8.
OBJECTIVES AND RATIONALE (NEED AND PURPOSE):
Fisheries acoustic surveys in the Benguela region have in the past only focused on
commercially important species such as sardine, anchovy, horse mackerel and round
herring (Barange et al. 1999, Boyer and Hampton 2001). Recently they have also been
used to estimate the biomass and target strength of jellyfish in Namibian waters (Brierley
et al. 2001) and have at times been used to correct estimates of hake biomass obtained
from bottom trawl surveys (Iilende et al. 2001). Given the synoptic nature of acoustic
surveys and improved technology available, these surveys are also ideal for estimating
the biomass of non-commercial species such as lanternfish and lightfish in a relatively
short time. Despite the perceived high biomass of meso-pelagic fish biomass in both the
northern and southern Benguela (Armstrong and Prosch (1991) and their associated
importance in the foodweb (Shannon and Jarre-Teichmann 1999), very little effort has,
however, been spent on estimating the biomass and target strength of meso-pelagic fish.
The combined biomass of the myctophid Lampanyctodes hectoris (the lanternfish) and
the sternoptychid Maurolicus muelleri (the lightfish) in the southern Benguela was
estimated by Armstrong and Prosch (1991) to be in the order of one million tons during
two surveys in 1983 and 1987. L hectoris is by far the most abundant myctophid in the
northern Benguela and is distributed over the outer shelf from Walvis Bay to the Orange
River and further south into South Africa's west coast area. The biomass of lantern fish in
79
BCLME project LMR/CF/03/08
the northern Benguela has previously been estimated at around 800 000 tons, although
negative biases such as under sampling during the day and net avoidance at night were
noted for this estimate (Hewitson and Cruikshank 1993). Several acoustic surveys
conducted each year in the Benguela region are restricted to the inner shelf area
(approximately 200 m isobath) and therefore not suitable for providing simultaneous
meso-pelagic fish biomass estimates, as the distributional range of both lanternfish and
lightfish extends out to at least a bottom depth of 500 m (Hulley and Prosch 1987).
Several attempts to model trophic flows in the southern and northern Benguela have had
to incorporate uncertainty about many of the parameter estimates in the mass balanced
models used (Jarre-Teichmann et al. 1998, Shannon et al. 2004, Roux and Shannon
2004). Whereas biomass estimates of the commercially important fish species are
available, data on meso-pelagic production and consumption have not been updated since
the mid 1980s. Results from trophic flow models of the region have, however, confirmed
that meso-pelagic fish play an important role in the foodweb of the Benguela, particularly
as a link between zooplankton and hake (Jarre-Teichmann et al. 1998, Shannon and Jarre-
Teichmann 1999). Apart from hake, meso-pelagic fish are also consumed by other
demersal fish and large horse mackerel, cephalopods, large pelagics such as Tuna and
snoek and even by seabirds.
In addition, round herring (Etrumeus whiteheadi) is currently not of commercial
importance in Namibia and there is limited information on the species from that region.
Round herring is also considered to be of little importance to the trophic flow of the
northern Benguela and is basically eliminated from the trophic flow models (Jarre-
Teichmann et al. 1998) due to there being little information available. During May 2004,
however, an acoustic survey of the LUCORC region by the R. S. Africana found
substantial amounts of round herring (260 000 tons) in the area between Lüderitz and the
Orange River (MCM unpublished data). These fish could be an important food source to
other top predators such as large pelagic fish (Shannon and Jarre-Teichmann 1999),
condrichthyans (Jarre-Teichmann et al. 1998), seals and seabirds (Crawford et al. 1991),
yet not enough information is available to include them into ecosystem models.
Given recent endeavors to move away from single-stock assessments procedures towards
an integrated ecosystems approach to fisheries management, some fundamental
uncertainties need to be addressed for several species groups. These include greater effort
to improve indices of biomass, consumption, predator selectivity and the variability in
these related to absolute abundances of prey species (Shannon et al. 2004, Roux and
Shannon 2004). This proposal aims to address some of the questions related to the meso-
pelagic species located in the northern and southern Benguela through a dedicated
acoustic survey, with bottom and midwater trawling for target identification, of the entire
shelf out to a depth of 500 m between Walvis Bay and Cape Point.
80
BCLME project LMR/CF/03/08
9.
KEY QUESTIONS AND RESEARCH APPROACH:
Key Questions
1.
What are the biomass and distribution patterns of meso-pelagic fish species in the
southern and Northern Benguela?
2.
Can the target strength of meso-pelagic fish species in the Benguela region be
measured in-situ using high resolution multi-frequency techniques?
3.
How do oceanographic variables influence the distribution and behaviour of
meso-pelagic fish species in the Benguela region?
4.
What oceanographic conditions characterise the spawning habitat of meso-pelagic
fish species in the Benguela region?
Research Approach
A dedicated three week acoustic survey is required on the Dr Fridtjof Nansen in either
July/August or September 2006 to answer the above key questions. Multi-frequency
acoustic data will be collected using a Simrad EK60 echo sounder at 38, 120 and 200 kHz
(MCM will supply GPTs if unavailable). The multi-sampler will be required for targeted
trawling of individual scattering layers. A temporary CUFES system will need to be set
up for egg collection. A comprehensive callibaration of the acoustic system will be
required prior to the survey. Specific objectives of the survey include:
· Collection of high resolution multi-frequency acoustic data for target strength
estimation of meso-pelagic fish species.
· Training of scientists from the region in disciplines such as acoustic survey
techniques, target strength estimation and spatial statistics.
· Estimation of the biomass and population length structure of lanternfish and lightfish
by means of echo-integration and targeted midwater/bottom trawling.
· Collection of data for the description of distribution and behaviour patterns of meso-
pelagic fish.
· Collection of meso-pelagic fish eggs through the use of a continuous underway fish
egg sampler (CUFES) for mapping of spawning habitat and investigation of the
influence of oceanographic variables on egg distribution patterns.
· Collection of environmental information to investigate the influence of oceanographic
variables on the distributional patterns of meso-pelagic fish species.
· Collection of biological data on reproduction and diet of meso-pelagic fish species
The above specific objectives may be expanded to incorporate other co-occuring species
found such as sardine, anchovy, round herring, horse mackerel, juvenile hake and pelagic
goby.
81
BCLME project LMR/CF/03/08
10.
PROPOSED WORK PLAN:
2006 1. Three week acoustic survey on Dr. Fridtjof Nansen (August/September)
with participants from MCM, NATMIRC and Oslo University.
2007 1. Participants of survey to get together in Cape Town or Walvis Bay to
analyse data from Survey and plan publication(s) (February/March)
2.
Authors involved in publications to meet in Cape Town or Walvis Bay for
writing of scientific publication(s) (August/September)
11.
END PRODUCTS OF THE PROJECT:
1. Biomass and Target strength estimates of the most important meso-pelagic
fish species in the Benguela region.
2. Distribution patterns, behavioural strategies and spawning habitat
characterisations of the most important Meso-pelagic fish species in the
Benguela region.
3. Revised biomass inputs for mass-balanced models of both the northern
and southern Benguela with associated changes in model outputs.
4. Publication of results in scientific literature
5. Training of inexperienced scientists from the region
6. Contribution of data for a PhD thesis (A. Staby)
12.
FINANCIAL REQUIREMENTS
2006
Running expenses
Communication expenses, computing consumables (eg DVD,s and CD's) N$ 5 000
stationery etc
Travel costs
One-way travel between Cape Town and Swakopmund for up to
N$ 25 000
MCM participants in cruise (including per diem)
One-way travel between Cape Town and Swakopmund for up to
N$ 15 000
4 Namibian participants in cruise (including per diem)
Return Travel for one student from Bergen University for participation
N$ 15 000
In cruise (including per diem)
Total
N$ 60 000
82
BCLME project LMR/CF/03/08
2007 (estimated)
Running expenses
Communication expenses, computing consumables (eg DVD,s and
N$ 5 000
CD's) stationery etc
Travel costs
Four return airfares between Cape Town and Swakopmund for
N$ 20 000
Data processing and finalization of publications
Accomodation and per diem for 2 participants from MCM to
N$ 20 000
Visit Swakopmund for two periods of 5 days
Four return airfares between Swakopmund and Cape Town for
N$ 20 000
Data processing and finalization of publications
Accomodation and per diem for 2 participants from NATMIRC to
N$ 20 000
Visit Cape Town for two periods of 5 days
Total
N$ 85 000
83
BCLME project LMR/CF/03/08
Annex 16: Contact details
Table A: Contact details of people approached for additional information on gobies and
mesopelagics
Contact person
Country
Institute/ Company
Position
Adddress
Angie
Namibia
National Marine
Senior
P O Box 912
Kanadjembo
Information & Research Biologist
Swakopmund, Namibia
Centre (NatMIRC)
Tel: +264 64 410 1000
Fax: +264 64 404 385
akanandjembo@mfmr.gov.na
Philomena Vaz
Angola
Instituto de Investigacao Biologist:
P O Bocx 2611
Velho
Marinha (IMM)
Pelagics
Luanda, Angola
Johan Augustyn
South
Marine & Coastal
Director:
Department of
Africa
Management (MCM)
Research
Environmental Affairs &
Tourism
Tel: +27 21 402 3102
Fax: +27 21 429 6977
augustyn@deat.gov.za
Tracy Fairweather South
Marine & Coastal
Senior
Marine & Coastal
Africa
Management
Oceanographer Management
Tel: +27 21 402 3256
Fax: +27 21 421 7406
tracey@deat.gov.za
Alexei Orlov
Russia
Russian Federal
Principal
17, V. Krasnoselskaya,
Research Institute of
Scientist
107140
Fisheries and
Tel: 264-91-43
Oceanography
Fax: 264-90-21
(VNIRO)
orlov@vniro.ru
Callie Jacobs
Namibia
Erongo Group
CEO
P O Box 1155
Walvis Bay, Namibia
Tel: +264 64 219 200
Fax: + 264 64 209 214
Cell: +264 81 124 8294
cjacobs@erongo.co.za
84











