



United Nations
Environment Programme
Chemicals
Nor
North America
th America
REGIONAL REPORT
RBA PTS REGIONAL REPOR
Regionally
Based
Assessment
T
of
Persistent
Available from:
UNEP Chemicals
11-13, chemin des Anémones
CH-1219 Châtelaine, GE
Switzerland
Phone : +41 22 917 1234
Fax : +41 22 797 3460
Substances
E-mail: chemicals@unep.ch
December 2002
http://www.chem.unep.ch
UNEP Chemicals is a part of UNEP's Technology, Industry and
Printed at United Nations, Geneva
Economics Division
GE.03-00147January 2003500
UNEP/CHEMICALS/2003/2
G l o b a l E n v i r o n m e n t F a c i l i t y
UNITED NATIONS
ENVIRONMENT
PROGRAMME
CHEMICALS
Regional y Based Assessment
of Persistent Toxic Substances
Canada, Mexico,
United States of America
NORTH AMERICA
REGIONAL REPORT
DECEMBER 2002
GLOBAL ENVIRONMENT FACILITY
This report was financed by the Global Environment Facility (GEF) through a global project with co-
financing from the Governments of Australia, France, Sweden, Switzerland and the United States of
America
This publication is produced within the framework of the Inter-Organization Programme for the Sound
Management of Chemicals (IOMC)
This publication is intended to serve as a guide. While the information provided is believed to be
accurate, UNEP disclaim any responsibility for the possible inaccuracies or omissions and
consequences, which may flow from them. UNEP nor any individual involved in the preparation of
this report shall be liable for any injury, loss, damage or prejudice of any kind that may be caused by
any persons who have acted based on their understanding of the information contained in this
publication.
The designations employed and the presentation of the material in this report do not imply the
expression of any opinion whatsoever on the part of the Secretariat of the United Nations of UNEP
concerning the legal status of any country, territory, city or area, or of its authorities, or concerning
the delimitation of its frontiers or bounaries.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC), was
established in 1995 by UNEP, ILO, FAO, WHO, UNIDO and OECD (Participating
Organizations), following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase coordination in the
field of chemical safety. In January 1998, UNITAR formally joined the IOMC as a Participating
Organization. The purpose of the IOMC is to promote coordination of the policies and
activities pursued by the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the environment.
Material in this publication may be freely quoted or reprinted but acknowledgement is requested
together with a reference to the document. A copy of the publication containing the quotation or
reprint should be sent to UNEP Chemicals.
UNEP
CHEMICALS
Available from:
UNEP Chemicals11-13, chemin des Anémones
CH-1219 Châtelaine, GE
Switzerland
Phone: +41 22 917 1234
Fax:
+41 22 797 3460
E-mail: chemicals@unep.ch
http://www.chem.unep.ch
UNEP Chemicals is a part of UNEP's Technology, Industry and Economics Division
ii
TABLE OF CONTENTS
Preface.................................................................................................................................................................... v
Executive Summary ............................................................................................................................................ vii
1. Introduction ....................................................................................................................................................... 1
1.1 Scope of the North American Regional Assessment..................................................................................... 1
1.2 Methodology ................................................................................................................................................. 2
1.3 General Definitions of Chemicals ................................................................................................................. 2
1.4 Definition of the UNEP Region of North America ..................................................................................... 14
1.5 Physical Setting ........................................................................................................................................... 14
1.6 References ................................................................................................................................................... 21
2. Source Characterization................................................................................................................................. 23
2.1. Background Information on PTS Sources.................................................................................................. 23
2.2 Production, Use and Emissions ................................................................................................................... 27
2.3 Hot Spots..................................................................................................................................................... 49
2.4 Data Gaps and Programme Needs............................................................................................................... 51
2.5 Conclusions ................................................................................................................................................. 52
2.6 References ................................................................................................................................................... 52
3. Environmental Levels, Toxicology and Ecotoxicology Patterns ................................................................. 56
3.1 Environmental Levels and Trends............................................................................................................... 56
3.2 Ecotoxicology of PTSs of Regional Concern.............................................................................................. 86
3.3 Toxicology, Human Levels and Health Effects .......................................................................................... 95
3.4 Data Gaps and Programme Needs............................................................................................................. 100
3.5 Conclusions ............................................................................................................................................... 102
3.6 References ................................................................................................................................................. 103
4. Major Pathways of Transport...................................................................................................................... 111
4.1 Introduction ............................................................................................................................................... 111
4.2 Overview of Existing Modelling Programmes and Projects ..................................................................... 112
4.3 The Atmosphere ........................................................................................................................................ 116
4.4 Freshwater ................................................................................................................................................. 116
4.5 Data Gaps .................................................................................................................................................. 117
4.6 Conclusions ............................................................................................................................................... 117
4.7 References ................................................................................................................................................. 118
5. Preliminary Assessment of the Regional Capacity and Need to Manage Persistent Toxic Substances 119
5.1 Introduction ............................................................................................................................................... 119
5.2 Monitoring Capacity ................................................................................................................................. 119
5.3 Existing regulations and management structures ...................................................................................... 123
5.4 Status of Enforcement and Compliance .................................................................................................... 128
5.5 Alternatives or Measures for Reduction.................................................................................................... 129
5.6 Technology Transfer ................................................................................................................................. 130
5.7 Identification of Needs .............................................................................................................................. 130
5.8 Conclusions ............................................................................................................................................... 131
5.9 References ................................................................................................................................................. 131
iii
6. Final Results and Recommendations........................................................................................................... 133
6.1 Identification of Barriers ........................................................................................................................... 133
6.2 Priorities and Recommendations for Future Activities ............................................................................. 134
ANNEX: Scoring for Prioritising PTS for Sources, Environmental Levels, Effects and Data gaps ........ 136
iv
PREFACE
Following the recommendations of the Intergovernmental Forum on Chemical Safety, the UNEP Governing
Council decided in February 1997 (Decision 19/13 C) that immediate international action should be initiated to
protect human health and the environment through measures which will reduce and/or eliminate the emissions
and discharges of an initial set of twelve persistent organic pollutants (POPs). Accordingly an
Intergovernmental Negotiating Committee (INC) was established with a mandate to prepare an international
legally binding instrument for implementing international action on certain persistent organic pollutants. These
series of negotiations have resulted in the adoption of the Stockholm Convention in 2001. The initial 12
substances fitting these categories that have been selected under the Stockholm Convention include: aldrin,
endrin, dieldrin, chlordane, DDT, toxaphene, mirex, heptachlor, hexachlorobenzene, PCBs, dioxins and furans.
Beside these 12, there are many other substances that satisfy the criteria listed above for which their sources,
environmental concentrations and effects are to be assessed.
Persistent toxic substances can be manufactured substances for use in various sectors of industry, pesticides, or
by-products of industrial processes and combustion. To date, their scientific assessment has largely
concentrated on specific local and/or regional environmental and health effects, in particular "hot spots" such as
the Great Lakes region of North America or the Baltic Sea.
Objectives
There is a need for a scientifically-based assessment of the nature and scale of the threats to the environment and
its resources posed by persistent toxic substances that will provide guidance to the international community
concerning the priorities for future remedial and preventive action. The assessment will lead to the
identification of priorities for intervention, and through application of a root cause analysis will attempt to
identify appropriate measures to control, reduce or eliminate releases of PTS, at national, regional or global
levels.
The objective of the project is to deliver a measure of the nature and comparative severity of damage and threats
posed at national, regional and ultimately at global levels by PTS. This will provide the GEF with a science-
based rationale for assigning priorities for action among and between chemical related environmental issues, and
to determine the extent to which differences in priority exist among regions.
Results
The project relies upon the collection and interpretation of existing data and information as the basis for the
assessment. No research will be undertaken to generate primary data, but projections will be made to fill
data/information gaps, and to predict threats to the environment. The proposed activities are designed to obtain
the following expected results:
1. Identification of major sources of PTS at the regional level;
2. Impact of PTS on the environment and human health;
3. Assessment of transboundary transport of PTS;
4. Assessment of the root causes of PTS related problems, and regional capacity to manage these problems;
5. Identification of regional priority PTS related environmental issues; and
6. Identification of PTS related priority environmental issues at the global level.
The outcome of this project will be a scientific assessment of the threats posed by persistent toxic substances to
the environment and human health. The activities to be undertaken in this project comprise an evaluation of the
sources of persistent toxic substances, their levels in the environment and consequent impact on biota and
humans, their modes of transport over a range of distances, the existing alternatives to their use and remediation
options, as well as the barriers that prevent their good management. The information and conclusions derived
from the 12 regional reports will be used to develop a global report on the state of these PTS in the environment.
Regional divisions
To achieve these results, the globe is divided into 12 regions namely: Arctic, North America, Europe,
Mediterranean, Sub-Saharan Africa, Indian Ocean, Central and North East Asia (Western North Pacific), South
East Asia and South Pacific, Pacific Islands, Central America and the Caribbean, Eastern and Western south
v
America, Antarctica. The twelve regions were selected based on obtaining geographical consistency while
trying to reside within financial constraints.
Management structure
The project is directed by the project manager who is situated at UNEP Chemicals in Geneva, Switzerland. A
Steering Group comprising of representatives of other relevant intergovernmental organizations along with
participation from industry and the non-governmental community is established to monitor the progress of the
project and provide direction for the project manager. An MOU was signed between UNEP Chemicals and the
Commission for Environmental Cooperation's (CEC) Secretariat in Montreal, Canada. Mr. Victor Shantora of
the Secretariat is the Regional Coordinator for Region II North America. Mr. Shantora has been assisted by a
team of experts. The co-ordinator and the regional team are responsible for implementing the project, managing
the report production and establishing priorities for the chemicals. Besides the 12 POPs from the Stockholm
Convention, the Steering Group selected the chemicals to be assessed by the regions. In addition to the 27
substances selected by the UNEP Steering Group, one additional substance was added to the Region II list by
the CEC Secretariat, namely perfluorooctane sulfonate (PFOS). This addition seems appropriate given the recent
release of the 362-page OECD scientific assessment report on PFOS based on which the OECD Chemical
Committee has concluded that "PFOS is persistent, bioaccumulative and toxic to mammalian species".
The material presented in this report is drawn from existing reports, scientific papers and assessments. This
report was prepared for the CEC Secretariat . The views contained herein do not necessarily reflect the views of
the governments of Canada, Mexico or the United States of America. At a priority setting workshop, October 1
and 2, 2002, experts from each country reviewed the draft report and offered their advice on priorities regarding
the threats and damages of these substances in Region II.
The project is not intended to generate new information but to rely on existing data and its assessment to arrive
at priorities for these substances. A broad and wide- ranging network of participants involving all sectors of
society was used for evaluation of the report drafts. Close cooperation with other intergovernmental
organizations such as UNECE, WHO, FAO, UNPD, World Bank and others was obtained. Most have
representatives on the Steering Group Committee that monitors the progress of the project and critically reviews
its implementation. Contributions were garnered from UNEP focal points, UNEP POPs focal points, national
focal points selected by the regional teams, industry, government agencies, research scientists and NGOs.
Project funding
The project costs approximately US$4.2 million funded mainly by the Global Environment Facility (GEF) with
sponsorship from countries including Australia, Canada, France, Germany, Sweden, Switzerland and the USA.
The North America regional report was partly funded by the North America Commission for Environmental
Cooperation. The project runs between September 2000 to April 2003 with the intention that the reports be
presented to the first meeting of the Conference of the Parties of the Stockholm Convention projected for
2003/4.
Report Production
In 2001, the United Nations Environmental Program (UNEP) asked the CEC) Secretariat Montreal, Canada, to
participate in a global assessment of PTS, in particular to produce the required UNEP report on PTSs for Region
II - North America. The production of this report was made possible through a contract between the CEC and
Global Change Strategies International (GCSI)-Natsource Inc., Ottawa, Canada. The responsible authority for
this contract was Mr. Victor Shantora, and Dr. Joanne O'Reilly, CEC Secretariat . The coordinator for this study
was Dr. Hans Martin, Senior Associate, GCSI-Natsource. Mr. Jorge Sanchez provided the Mexican portion of
the material in Chapters 2 and 3. Mr. Roy Hickman prepared the Human Health component of Chapter 3. Mr.
Tony Clarke wrote Chapter 5 on Regional Capacity. Ms. Stephanie Martin was responsible for research. Mr.
Padro Colucci assisted with the translation of Spanish documents and the organization of material. Chapter 6,
Final Results and Recommendations, was formulated based on input from experts at the priority setting
workshop, October 1 and 2, 2002. Dra. Leonor Cedillo Becerril and her colleagues in Mexico are acknowledged
for their significant contribution to the Mexican component of the report.
vi
Hans Martin, Ph. D.
Global Change Strategies International Natsource
Ottawa, Canada
December 2002
EXECUTIVE SUMMARY
Introduction
The guidelines for the United Nations Environment Programme (UNEP), Regionally Based Assessment (RBA)
of Persistent Toxic Substances (PTSs) define the boundaries of each region and provide a list of priority
persistent toxic chemicals (PTSs) and a report outline. Each region reports on those chemicals which are
important in that region.
This report on UNEP Region II was produced by the Commission for Environmental Cooperation (Montreal)
through a contract with Global Change Strategies International Inc. (Ottawa).
The purpose of this UNEP assessment is to provide the Global Environmental Facility (GEF) with a science-
based rationale for assigning priorities for action among and between chemical related environmental issues, and
to determine the extent to which differences in priority exist among regions.
This document was produced over a period of four months and is based entirely on published information in
reports and the scientific literature with supporting references as noted in the text.
Conclusions
Sources
The national substance inventories, NPRI and TRI, within the region have become valuable databases. However,
the national database in Mexico is still in its developmental stage while the national databases in Canada and the
USA have intrinsic weaknesses, including:
· They do not address all sources and do not include all important substances.
· They do not identify all on-site releases and off-site transfers from a facility.
· Some releases are estimated not measured.
· They do not indicate the ultimate environmental fate of materials, which transporting facilities release or
ship off-site for disposal or other disposition.
· They do not provide information on the toxicity or potential health effects of substances which reporting
facilities release or transfer.
· They do not identify exposure risk to human or ecological populations from substances released or
transferred by reporting facilities.
In Mexico, many required databases are limited or lacking. Access to information is difficult requiring intensive
searches to determine, when possible, the character, quantities and movements of PTSs. Cicoplafest's efficient
regulation and coordination of the handling and use of toxic substances has not been achieved for a number of
reasons. There should be an easy-to-use information system to provide data and statistics on PTS handling, as
well as pollution prevention measures, health risks and individual emergencies and disasters. Major
agrochemical producers should have greater involvement in the management of chemicals.
Procedures and criteria should be developed and applied to prioritize PTSs, including concepts such as handling,
movement, uses, health and environmental effects, etc.
Pathways
Atmospheric pathways are important in the overall context of the movement of contaminants in North America.
However, our understanding of these pathways is very uneven. Movements into and out of the Great Lakes
basin are well understood. This is not the case for pathways: into the region from the west; within the mountain
vii
ecosystems; and out of the central agricultural sub-region. The pathways in Mexico have in large part not been
studied.
With the suite of models available and under development, detailed assessments can be made of both local and
regional movements of PTSs. These assessments are not possible everywhere because of a lack of basic input
data (such as emission inventories) and/or institutional capacity.
Trilateral collaboration is not sufficiently developed to address regional scale issues concerning the transport of
PTSs.
Contamination levels, trends and effects
For much of North America, there is little information on the environmental levels, trends and consequences of
PTSs. The high quality and extensive coverage of research in the Great Lakes Basin stands in sharp contrast
with the rest of the region.
The Great Lakes: Three decades ago elevated levels of a wide range of PTSs were associated with an array of
impacts on wild life and risk to human health. Because of extensive remedial activities since the 1970's there
have been significant environmental improvement as demonstrated by recoveries in reproductive success and
increases in population for most of the affected, fish-eating, bird species. In 1991, concentrations of toxic
chemicals in the open waters of the Great Lakes are well below Canadian and international drinking water
standards.
Though the temporal trend in annual flow of pesticides into the five Great Lakes is generally decreasing, the
presence of toxic substances in the Great Lakes basin continues to be a significant concern for both wild life and
human health. Contaminant-related restrictions on the harvest of many commercial fish species are still in place
along with fish consumption advisories. Downward fluxes for in-use pesticides, such as -HCH, and PAHs
account for the highest fluxes into the lakes. The exchange fluxes for all PAHs show no real temporal trend.
In the Great Lakes, volatilization (upward) fluxes for banned pesticides are almost 10 times greater than those
for in-use pesticides. The trend in gas exchange of PCBs, Dieldrin and p,p'-DDE has been in the direction of net
volatilization indicating that the lakes are acting as sources.
Levels of PCBs and DDT have declined significantly in top predators. For example, continued decline in PCBs
in herring gull and osprey eggs reflects lower emissions following controls on open uses. Declines in Great
Lakes lake trout and walleye have not been as dramatic especially since the mid-1980s reflecting continued
emissions from urban areas and recycling of contaminants within the lakes.
The Region: Despite the declining levels, the interim guideline for PCB of 0.32 pg TEQ/g ww, designed to
protect Canadian wildlife that consume fish and shellfish, is routinely exceeded by both predator and forage fish
in many areas.
Levels of mercury, unlike the PCBs and DDT, have increased in the past 20 years in fish-eating birds and
mammals, particularly near urban centres. The interim guideline of Hg of 22 ng/g ww for protection of fish
consuming wildlife is exceeded in almost all fish measured to date in Canada. However, average mercury
concentrations in insectivorous fish such as lake whitefish and white sucker are well below this guideline.
From sediment studies it has been shown that urban areas are an important sources of many of PTSs e.g. PCBs,
chlorobenzenes and PBDEs (such as BDE209).
Current use OCs, endosulfan and lindane and high volume herbicides atrazine are reaching remote lakes. Recent
observations suggest that concentrations as low as 100 ng/L affect ecologically important aquatic systems. The
remote lakes could have levels approaching that threshold.
A lake water and sediment survey (1998 2001) across the north-eastern part of the region, reported on a
number of new potentially persistent chemicals not previously identified in remote lake surface waters, such as
dacthal, the fungicides flutriafol and chlorothalonil, deca-bromobiphenyl ether (a PBDE) and the PFOS
perfluorooactanoic acid, a product recently removed from the market by its manufacturer, 3M Corp. Some of
these chemicals are not part of this assessment.
viii
Recent studies of PCB, CDDs and CDFs have shown that persons who ate Great Lakes sport fish for more than
15 years have two to four times higher levels in their serum than non-fisheaters .
A 1999 review of 130 previous studies of global trends in average levels of DDT in breast milk shows a
downward trend in DDT concentrations in breast milk since about 1970. For the U.S. and Canada, the data
suggest an 11% to 21% per year reduction in average levels of DDT in breast milk since 1975.
Dieldrin, DDT and metabolites in breast milk samples from the Canadian population have shown declines over
the past three decades.
Emerging Chemicals of Concern
Growing evidence of the occurrence and potential impacts of new chemicals suggest that detailed assessments
should be conducted on flame retardants (PBDEs, PBBS and TBBPA), short chain chlorinated paraffins,
perfluorooctane sulfonate (PFOS), Polychlorinated naphthalenes (PCNs) and alkylphenol ethoxylates (APEs).
Detailed studies of environmental concentrations and associated observed effects are rare. In the case or
emerging PTSs, basic ecotoxicological data is often not available.
Gaps in current understanding
The lack of appropriate research programs is not the only barrier to the advancement of our common
understanding of the occurrence and consequences of PTSs in the region. The current region-wide study effort is
determined by broader considerations. There are basic economic, social and institutional barriers, such as lack of
funding, of incentives, of communication, of public participation and of information. The three countries have
differences in governmental models, cultural practices and legislation and regulatory processes.
Specific barriers to the advancement of our common understanding include:
Information
In Mexico, the occurrence of PTSs and their levels in all environmental compartments are practically unknown.
The majority of the compounds remain unstudied in the Region. For "new" chemicals, basic physical properties
and toxicology information is not available as well as analytical methods information.
Large parts of the Region are unstudied, including, the mountainous sub-region bounding the western edge of
the Region and coastal and riverine areas.
Training
Mexican participants often do not have sufficient training to make use of existing databases and to gather
appropriate data.
Research
Collaborative tri-national research and monitoring programs on PTSs with a continental focus are rare. They are
needed to ensure that information and expertise on sources, environmental levels, temporal trends, impacts,
modeling, etc. are available and are shared.
Uniformity of analytical methodologies is required.
Commitment to long-term research and monitoring programs is essential if trends in PTSs resulting from UN
ECE and UNEP agreements are to be detected and evaluated.
Little is known of the toxicological significance of constant exposure of organisms to low levels of chemicals.
Little is known about the potential effects of mixtures.
The development and application of regionally specific models for Mexico has not occurred.
Capacity
The community education process is weak.
Technology transfer/exchange agreements which promote compliance with national programs and regulations,
voluntary measures and harmonization in the three North American countries are needed.
ix
Recommendations
During the review of this report the following key priority areas were identified:
1. Emerging
PTSs:
· North America should leverage existing capacity and focus on research/assessment of emerging
PTSs.
2.
Knowledge and Technology Transfer in the Regions:
· Networks of scientists working together to address
o Scientific exchanges
o Emerging contaminants
o Technology transfer
o Information exchange and the aligning the procedures for assessment
3.
Ratification of the Stockholm Convention in North America:
· Tri-national ratification
· Develop national implementation plans with milestones (e.g. US manufacturers no longer exporting
POPs substances)
o Need program evaluation criteria
o Need clear roles and responsibility
4.
Addressing research and monitoring needs and data gaps related to:
· Sources, particularly in Mexico
· The physical/chemical properties of PTSs,
· Emission inventories including the quantification of sources, sinks and reservoirs
· Spatial variability of PTSs levels for an larger number of chemicals
· Exposure pathways in food, agriculture and aquaculture for exposed populations and subsistence
food production systems.
· Open Burning including developing a definition, taking a leadership role and encouraging a global
campaign.
5.
Improving the Mexican Infrastructure:
· Complete a national information structure for:
o Emissions, sources, sinks and reservoirs
o Areas with severe degradation
o Monitoring of contaminants in various compartments
· Develop analytical capacity to the point where comparable data and information exists among the
three countries.
6.
Creating a North American regional pollution prevention program:
· Include a framework for public education, technology transfer, and education in best practices.
· Encourage communication between existing infrastructures.
· A specific strategy needed for Mexico that will use and adopt technologies available in Canada and
the U.S.
· In areas where we have confidence now, take action to control and reduce.
7.
Public education in North America involving:
· NGO's
· Appropriate levels of government
· A focus on children, women and indigenous peoples.
x
1. INTRODUCTION
1.1 Scope of the North American Regional Assessment
This report has been reviewed by a group of North American stakeholders, which acted in place of the regional
committee and experts group established in other regions. The review took place at a two-day workshop held in
Montreal, October, 2002. At this meeting, experts were also asked to undertake a quantitative scoring analysis of
the selected chemicals (See Annex). This was done to provide a measure of the relative priority between
chemicals under the specific compartments considered in the report and also for Data Gaps. The results of the
scoring reflect only the views of the experts gathered and should be considered in this light. The final text was
subsequently prepared.
This is the first comprehensive North American assessment of its kind.
1.1.1 Existing Assessments
In North America, there have been a number of sub-regional assessments conducted over the past several
decades, particularly in the Great Lakes basin. These studies have included a large number of UNEP chemicals.
In the case of Mexico, programs to assess the existence and consequences of persistent toxic substances are
more recent and, as much as possible, are reflected in the following chapters.
1.1.2 Interregional Links and Collaboration
The preparation of this report was substantially assisted by the already existing draft RBA report provided by
UNEP Region I, the Arctic. There is an obvious connection between Regions I and II, since Canada and the
United States have territory in both regions. Canadian scientific laboratories, regulatory bodies, and political
centres are common to both regions. The same situation applies to the U.S. in reference to its Arctic state,
Alaska.
Links with other regions have been less formal. The writers of this report are grateful to UNEP Region IV for
providing the standard set of definitions of the chemicals. In addition, a draft of the UNEP Region III report
provided a useful guide on the treatment of some of the material. During the preparation of Chapter 5 (on
regional capacity) and the priority tables located in the annex, extensive inter-regional discussions and
collaboration occurred.
1.1.3 Use of Published Material
In most of the UNEP regions, the assessment reports were produced by teams of specialists, conversant with all
aspects of the issue. In the case of this report, the material presented is drawn from existing reports, scientific
papers and assessments. The small group of authors/editors relied substantially on the authority of the published
material consulted. In order to avoid misrepresentation and inaccurate interpretation, material from existing
documents has been quoted verbatim throughout this report. The use of published material in this way is not
intended to imply authorship by the writers, but is done in order to ensure precision in reporting. The authors
believe that this approach has ensured an authoritative documentation of the occurrence and consequences of
PTSs in UNEP Region II. Occasionally, chemicals in addition to those covered in this report were part of the
published data. Inclusion of information about these chemicals is not meant to imply that they have PTS
characteristics. On occasion, the text and figures cited from the scientific literature may include references to
chemicals which are not part of this assessment, for example in Section 3.1.2.4.4. Reference to these chemicals,
which are not defined in this document and are not part of this study, does not imply that they are or should be
included in this assessment.
1.1.4 Omissions / Weaknesses
The greatest weakness in the process of producing this draft report was the limitation on resources and time (13
weeks). There was a constant risk that important information might be overlooked. This situation applied
particularly to Mexico where the assessment and management of PTSs is developing rapidly. It has been
difficult to reflect these changes and to maintain contact with new scientific information, policy and recent
achievements.
1
1.2 Methodology
The overall objective of the UNEP-GEF PTSs assessment project is to deliver a comprehensive regionally based
assessment of the occurrence, damage and threats posed by PTSs worldwide and to develop priorities for
specific chemicals at the regional level. These priorities will provide guidance for future interventions on the
most important and pressing issues. Each of the twelve Regional Reports includes the identification of the
sources of PTSs, their levels and trends in humans and the environment, their impacts, their transport pathways,
and an assessment of society's capacity to manage these problems.
In North America, a number of PTS assessments have been conducted or are ongoing. They are regional or
bilateral and, more recently through the efforts of the Commission for Environmental Cooperation (CEC),
trilateral. As a consequence, there is a considerable amount of documentation available, particularly in Canada
and the U.S. However, across the UNEP Region II as a whole, the examination and reporting on PTSs is very
irregular. It will quickly become evident that the Great Lakes Basin is a key component of the UNEP Region II
assessment. This focus is justified given the importance of the basin and the extent of documentation available.
For other important sub-regions, information is lacking and consequently this report may be incomplete.
The preparation of this report has relied mainly on the editing and synthesis of the existing published material.
The procedure did not include some of the prescribed UNEP activities that occurred in other UNEP regions such
as striking regional committees, establishing teams of experts, holding organizational meetings, gathering data
and undertaking analyses.
1.3 General Definitions of Chemicals
The UNEP spent considerable time and effort in deciding which chemicals should be included in this project. It
was agreed that there was no simple wording to define persistent toxic substances but that these substances
shared the following characteristics: organic (including organometalic) substances; slowly degraded in the
environment; accumulating in biota; and toxic.
In addition, the UNEP experts agreed that there were cases where substances with moderate inherent persistence
were continuously being released in large quantities over substantial areas. These releases might lead to
continuous exposure of organisms that would simulate that of more persistent substances. These less persistent
substances should, therefore, also be considered in the project. Among the toxic effects considered for PTSs,
endocrine disruption should be noted.
The 12 persistent organic pollutants (POPS) which are the subject of the recently signed Stockholm Convention
are regarded as contaminants of most immediate concern. During the UNEP preparation phase, other groups of
chemicals were suggested and discussed thoroughly. In addition, the inclusion of organometalic substances was
considered and highest priority was given to organomercury and organotin.
In the end, 26 chemicals or groups of chemicals were placed on the UNEP list: the 12 priority persistent organic
pollutants of global interest in the Stockholm Convention (aldrin, endrin, dieldrin, chlordane, DDT, heptachlor,
mirex, toxaphene, hexachlorobenzene, PCBs, dioxins, and furans), three organometals (organotin,
organomercury and organolead compounds) and eleven chemicals (HCH, PAHs, endosulphan,
pentachlorophenol, phthalates, PBDE, chlordecone, octylphenols, nonylphenols, atrazine and short chained
chlorinated paraffins) which are receiving growing attention. Of this last group of chemicals, all are considered
of relevance in North America and are included in the regionally specific PTSs discussion in Section 2.2.4. In
addition, one additional chemical, perfluorooctane sulfonate (PFOS), has been added to the UNEP list. It is
considered of relevance in North America. Thus the North American regional assessment considers 27
chemicals.
Occasionally the term POPs is used in this assessment to refer to the subset of chemicals identified in the
Convention. This usage is a reflection on the use of the term `POPs' in the relevant source documents and
citations and should cause no confusion.
In the following section, the 12 Stockholm compounds are separated into three groups, namely, pesticides
(nine), industrial chemicals (one) and unintended by-products (two). They are followed by descriptions of the
PTSs of regionally interest.
2
1.3.1 Pesticides
1.3.1.1 Aldrin
Chemical Name: 1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4-endo,exo-5,8-dimethanonaphthalene
(C12H8Cl6).
CAS Number: 309-00-2
Properties: Solubility in water: 27 µg/L at 25°C; vapour pressure: 2.31 x 10-5 mm Hg at 20°C; log KOW: 5.17-
7.4.
Discovery/Uses: It has been manufactured commercially since 1950, and used throughout the world up to the
early 1970s to control soil pests such as corn rootworm, wireworms, rice water weevil, and grasshoppers. It has
also been used to protect wooden structures from termites.
Persistence/Fate: Readily metabolized to dieldrin by both plants and animals. Biodegradation is expected to be
slow and it binds strongly to soil particles, and is resistant to leaching into groundwater. Aldrin was classified as
moderately persistent with half-life in soil ranging from 20-100 days.
Toxicity: Aldrin is toxic to humans; the lethal dose for an adult has been estimated to be about 80 mg/kg body
weight. The acute oral LD50 in laboratory animals is in the range of 33 mg/kg body weight for guinea pigs to
320 mg/kg body weight for hamsters. The toxicity of aldrin to aquatic organisms is quite variable, with aquatic
insects being the most sensitive group of invertebrates. The 96-h LC50 values range from 1-200 µg/L for insects,
and from 2.2-53 µg/L for fish. The maximum residue limits in food recommended by FAO/WHO varies from
0.006 mg/kg milk fat to 0.2 mg/kg meat fat. Water quality criteria between 0.1 to 180 µg/L have been published.
1.3.1.2 Dieldrin
Chemical Name: 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydroexo-1,4-endo-5,8-
dimethanonaphthalene (C12H8Cl6O).
CAS Number: 60-57-1
Properties: Solubility in water: 140 µg/L at 20°C; vapour pressure: 1.78 x 10-7 mm Hg at 20°C; log KOW: 3.69-
6.2.
Discovery/Uses: It appeared in 1948 after World War II and used mainly for the control of soil insects such as
corn rootworms, wireworms and cutworms.
Persistence/Fate: It is highly persistent in soils, with a half-life of 3-4 years in temperate climates, and
bioconcentrates in organisms.
Toxicity: The acute toxicity for fish is high (LC50 between 1.1 and 41 mg/L) and moderate for mammals (LD50
in mouse and rat ranging from 40 to 70 mg/kg body weight). Aldrin and dieldrin mainly affect the central
nervous system but there is no direct evidence that they cause cancer in humans. The maximum residue limits in
food recommended by FAO/WHO varies from 0.006 mg/kg milk fat and 0.2 mg/kg poultry fat. Water quality
criteria between 0.1 to 18 µg/L have been published.
1.3.1.3 Endrin
Chemical Name: 3,4,5,6,9,9-Hexachloro-1a,2,2a,3,6,6a,7,7a-octahydro-2,7:3,6-dimethanonaphth[2,3-b]oxirene
(C12H8Cl6O).
CAS Number: 72-20-8
Properties: Solubility in water: 220-260 µg/L at 25 °C; vapour pressure: 7 x 10-7 mm Hg at 25°C; log KOW:
3.21-5.34
Discovery/Uses: It has been used since the 50s against a wide range of agricultural pests, mostly on cotton but
also on rice, sugar cane, maize and other crops. It has also been used as a rodenticide.
Persistence/Fate: Is highly persistent in soils (half-lives of up to 12 years have been reported in some cases).
Bioconcentration factors of 14 to 18,000 have been recorded in fish, after continuous exposure.
3
Toxicity: Endrin is very toxic to fish, aquatic invertebrates and phytoplankton; the LC50 values are mostly less
than 1 µg/L. The acute toxicity is high in laboratory animals, with LD50 values of 3-43 mg/kg, and a dermal
LD50 of 5-20 mg/kg in rats. Long term toxicity in the rat has been studied over two years and a NOEL of 0.05
mg/kg bw/day was found.
1.3.1.4 Chlordane
Chemical Name: 1,2,4,5,6,7,8,8-Octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methanoindene (C10H6Cl8).
CAS Number: 57-74-9
Properties: Solubility in water: 56 µg/L at 25°C; vapour pressure: 0.98 x 10-5 mm Hg at 25 °C; log KOW: 6.00.
Discovery/Uses: Chlordane appeared in 1945 and was used primarily as an insecticide for control of
cockroaches, ants, termites, and other household pests. Technical chlordane is a mixture of at least 120
compounds. Of these, 60-75% are chlordane isomers, the remainder being related to endo-compounds including
heptachlor, nonachlor, diels-alder adduct of cyclopentadiene and penta/hexa/octachlorocyclopentadienes.
Persistence/Fate: Chlordane is highly persistent in soils with a half-life of about 4 years. Its persistence and
high partition coefficient promotes binding to aquatic sediments and bioconcentration in organisms.
Toxicity: LC50 from 0.4 mg/L (pink shrimp) to 90 mg/L (rainbow trout) have been reported for aquatic
organisms. The acute toxicity for mammals is moderate with an LD50 in rat of 200-590 mg/kg body weight (19.1
mg/kg body weight for oxychlordane). The maximum residue limits for chlordane in food are, according to
FAO/WHO between 0.002 mg/kg milk fat and 0.5 mg/kg poultry fat. Water quality criteria of 1.5 to 6 µg/L have
been published. Chlordane has been classified as a substance for which there is evidence of endocrine disruption
in an intact organism and possible carcinogenicity to humans.
1.3.1.5 Heptachlor
Chemical Name: 1,4,5,6,7,8,8-Heptachloro-3a,4,7,7a-tetrahydro-4,7-methanoindene (C10H5Cl7).
CAS Number: 76-44-8
Properties: Solubility in water: 180 µg/L at 25°C; vapour pressure: 0.3 x 10-5mm Hg at 20°C; log KOW: 4.4-5.5.
Production/Uses: Heptachlor is used primarily against soil insects and termites, but also against cotton insects,
grasshoppers, and malaria mosquitoes. Heptachlor epoxide is a more stable breakdown product of heptachlor.
Persistence/Fate: Heptachlor is metabolised in soils, plants and animals to heptachlor epoxide, which is more
stable in biological systems and is carcinogenic. The half-life of heptachlor in soil is in temperate regions 0.75
2 years. Its high partition coefficient provides the necessary conditions for bioconcentrating in organisms.
Toxicity: The acute toxicity of heptachlor to mammals is moderate (LD50 values between 40 and 119 mg/kg
have been published). The toxicity to aquatic organisms is higher and LC50 values down to 0.11 µg/L have been
found for pink shrimp. Limited information is available on the effects in humans and studies are inconclusive
regarding heptachlor and cancer. The maximum residue levels recommended by FAO/WHO are between 0.006
mg/kg milk fat and 0.2 mg/kg meat or poultry fat.
1.3.1.6 Dichlorodiphenyltrichloroethane (DDT)
Chemical Name: 1,1,1-Trichloro-2,2-bis-(4-chlorophenyl)-ethane (C14H9Cl5).
CAS Number: 50-29-2.
Properties: Solubility in water: 1.2-5.5 µg/L at 25°C; vapour pressure: 0.02 x 10-5 mm Hg at 20°C; log KOW:
6.19 for pp-DDT, 5.5 for pp-DDD and 5.7 for pp-DDE.
Discovery/Use: DDT appeared for use during World War II to control insects that spread diseases like malaria,
dengue fever and typhus. Following this, it was widely used on a variety of agricultural crops. The technical
product is a mixture of about 85% pp''-DDT and 15% op'-DDT isomers.
4
Persistence/Fate: DDT is highly persistent in soils with a half-life of about 1.1 to 3.4 years. It also exhibits high
bioconcentration factors (in the order of 50000 for fish and 500000 for bivalves). In the environment, the
product is metabolized mainly to DDD and DDE.
Toxicity: The lowest dietary concentration of DDT reported to cause egg shell thinning was 0.6 mg/kg for the
black duck. LC50 of 1.5 mg/L for largemouth bass and 56 mg/L for guppy have been reported. The acute toxicity
of DDT for mammals is moderate with an LD50 in rat of 113-118 mg/kg body weight. DDT has been shown to
have an estrogen-like activity and possible carcinogenic activity in humans. The maximum residue level in food
recommended by WHO/FAO, ranges from 0.02 mg/kg milk fat to 5 mg/kg meat fat. Maximum permissible
DDT residue levels in drinking water (WHO) is 1.0 µg/L.
1.3.1.7 Toxaphene
Chemical Name: Polychlorinated bornanes and camphenes (C10H10Cl8).
CAS Number: 8001-35-2
Properties: Solubility in water: 550 µg/L at 20°C; vapour pressure: 0.2-0.4 mm Hg (?) at 25°C; log KOW: 3.23-
5.50.
Discovery/Uses: Toxaphene has been in use since 1949 as a nonsystemic insecticide with some acaricidal
activity, primarily on cotton, cereal grains fruits, nuts and vegetables. It was also used to control livestock
ectoparasites such as lice, flies, ticks, mange, and scab mites. The technical product is a complex mixture of
over 300 congeners, containing 67-69% chlorine by weight.
Persistence/Fate: Toxaphene has a half life in soil from 100 days up to 12 years. It has been shown to
bioconcentrate in aquatic organisms (BCF of 4247 in mosquito fish and 76000 in brook trout).
Toxicity: Toxaphene is highly toxic in fish, with 96-hour LC50 values in the range of 1.8 µg/L in rainbow trout
to 22 µg/L in bluegill. Long term exposure to 0.5 µg/L reduced egg viability to zero. The acute oral toxicity is in
the range of 49 mg/kg body weight in dogs to 365 mg/kg in guinea pigs. In long term studies NOEL in rats was
0.35 mg/kg bw/day, LD50 ranging from 60 to 293 mg/kg bw. For toxaphene, there exists strong evidence of the
potential for endocrine disruption. Toxaphene is carcinogenic in mice and rats and is of carcinogenic risk to
humans, with a cancer potency factor of 1.1 mg/kg/day for oral exposure.
1.3.1.8 Mirex
Chemical Name: 1,1a,2,2,3,3a,4,5,5a,5b,6-Dodecachloroacta-hydro-1,3,4-metheno-1H-cyclobuta[cd]pentalene
(C10Cl12).
CAS Number: 2385-85-5
Properties: Solubility in water: 0.07 µg/L at 25°C; vapour pressure: 3 x 10-7 mm Hg at 25°C; log KOW: 5.28.
Discovery/Uses: The use in pesticide formulations started in the mid 1950s largely focused on the control of
ants. It is also a fire retardant for plastics, rubber, paint, paper and electrical goods. Technical grade preparations
of mirex contain 95.19% mirex and 2.58% chlordecone, the rest being unspecified. Mirex is also used to refer to
bait comprising corncob grits, soya bean oil, and mirex.
Persistence/Fate: Mirex is considered to be one of the most stable and persistent pesticides, with a half-life in
soils of up to 10 years. Bioconcentration factors of 2600 and 51400 have been observed in pink shrimp and
fathead minnows, respectively. It is capable of undergoing long-range transport due to its relative volatility
(VPL = 4.76 Pa; H = 52 Pa m 3 /mol).
Toxicity: The acute toxicity of Mirex for mammals is moderate with an LD50 in rat of 235 mg/kg and dermal
toxicity in rabbits of 80 mg/kg. Mirex is also toxic to fish and can affect their behaviour (LC50 (96 hr) from 0.2
to 30 mg/L for rainbow trout and bluegill, respectively). Delayed mortality of crustaceans occurred at 1 µg/L
exposure levels. There is evidence of its potential for endocrine disruption and possibly carcinogenic risk to
humans.
1.3.1.9 Hexachlorobenzene (HCB)
Chemical Name: Hexaclorobenzene (C6H6).
5
CAS Number: 118-74-1
Properties: Solubility in water: 50 µg/L at 20°C; vapour pressure: 1.09 x 10-5 mm Hg at 20°C; log KOW: 3.93-
6.42.
Discovery/Uses: It was first introduced in 1945 as fungicide for seed treatments of grain crops, and used to
make fireworks, ammunition, and synthetic rubber. Today it is mainly a by-product in the production of a large
number of chlorinated compounds, particularly lower chlorinated benzenes, solvents and several pesticides.
HCB is emitted to the atmosphere in flue gases generated by waste incineration facilities and metallurgical
industries.
Persistence/Fate: HCB has an estimated `field half-life' of 2.7-5.7 years. HCB has a relatively high
bioaccumulation potential and long half-life in biota.
Toxicity: LC50 for fish varies between 50 and 200 µg/L. The acute toxicity of HCB is low with LD50 values of
3.5 mg/g for rats. Mild effects of the [rat] liver have been observed at a daily dose of 0.25 mg HCB/kg bw. HCB
is known to cause liver disease in humans (porphyria cutanea tarda) and has been classified as a possible
carcinogen to humans by IARC.
1.3.2 Industrial compounds
1.3.2.1 Polychlorinated biphenyls (PCBs)
Chemical Name: Polychlorinated biphenyls (C12H(10-n)Cln, where n is within the range of 1-10).
CAS Number: Various (e.g. for Aroclor 1242, CAS No.: 53469-21-9; for Aroclor 1254, CAS No.: 11097-69-
1);
Properties: Water solubility decreases with increasing chlorination: 0.01 to 0.0001 µg/L at 25°C; vapour
pressure: 1.6-0.003 x 10-6 mm Hg at 20°C; log KOW : 4.3-8.26.
Discovery/Uses: PCBs were introduced in 1929 and were manufactured in different countries under various
trade names (e.g., Aroclor, Clophen, and Phenoclor). They are chemically stable and heat resistant, and were
used worldwide as transformer and capacitor oils, hydraulic and heat exchange fluids, and lubricating and
cutting oils. Theoretically, a total of 209 possible chlorinated biphenyl congeners exist, but only about 130 of
these are likely to occur in commercial products.
Persistence/Fate: Most PCB congeners, particularly those lacking adjacent unsubstituted positions on the
biphenyl rings (e.g., 2,4,5-, 2,3,5- or 2,3,6-substituted on both rings) are extremely persistent in the environment.
They are estimated to have half-lives ranging from three weeks to two years in air and, with the exception of
mono- and di-chlorobiphenyls, more than six years in aerobic soils and sediments. PCBs also have extremely
long half-lives in adult fish, for example, an eight-year study of eels found that the half-life of CB153 was more
than ten years.
Toxicity: LC50 for the larval stages of rainbow trout is 0.32 µg/L with a NOEL of 0.01 µg/L. The acute toxicity
of PCB in mammals is generally low and LD50 values in rat of 1 g/kg bw. IARC has concluded that PCBs are
carcinogenic to laboratory animals and probably also for humans. They have also been classified as substances
for which there is evidence of endocrine disruption in an intact organism.
1.3.3 Unintended by-products
1.3.3.1 Polychlorinated dibenzo-p-dioxins (PCDDs) and Polychlorinated dibenzofurans (PCDFs)
Chemical Name: PCDDs (C12H(8-n)ClnO2) and PCDFs (C12H(8-n)ClnO) may contain between 1 and 8 chlorine
atoms. Dioxins and furans have 75 and 135 possible positional isomers, respectively.
CAS Number:
Properties: Solubility in water: in the range 550 0.07 ng/L at 25°C; vapour pressure: 2 0.007 x 10-6 mm Hg
at 20°C; log KOW: in the range 6.60 8.20 for tetra- to octa-substituted congeners.
Discovery/Uses: They are by-products resulting from the production of other chemicals and from the low-
temperature combustion and incineration processes. They have no known use.
6
Persistence/Fate: PCDD/Fs are characterized by their lipophilicity, semi-volatility and resistance to degradation
(half life of TCDD in soil of 10-12 years) and to long-range transport. They are also known for their ability to
bio-concentrate and biomagnify under typical environmental conditions.
Toxicity: The toxicological effects reported refers to the 2,3,7,8-substituted compounds (17 congeners) that are
agonist for the AhR. All the 2,3,7,8-substituted PCDDs and PCDFs plus dioxin-like PCBs (DLPCBs) (with no
chlorine substitution at the ortho positions) show the same type of biological and toxic response. Possible effects
include dermal toxicity, immunotoxicity, reproductive effects and teratogenicity, endocrine disruption and
carcinogenicity. At the present time, the only persistent effect associated with dioxin exposure in humans is
chloracne. The most sensitive groups are fetus and neonatal infants.
Effects on the immune systems in the mouse have been found at doses of 10 ng/kg bw/day, while reproductive
effects were seen in rhesus monkeys at 1-2 ng/kg bw/day. Biochemical effects have been seen in rats down to
0.1 ng/kg bw/day. In a re-evaluation of the TDI for dioxins, furans (and planar PCB), the WHO decided to
recommend a range of 1-4 TEQ pg/kg bw, although more recently the acceptable intake value has been set
monthly at 1-70 TEQ pg/kg bw.
1.3.4 Regionally specific PTSs
1.3.4.1 Atrazine
Chemical Name: 2-Chloro-4-(ethlamino)-6-(isopropylamino)-s-triazine (C10H6Cl8).
CAS Number: 19-12-24-9
Properties: Solubility in water: 28 mg/L at 20°C; vapour pressure: 3.0 x 10-7 mm Hg at 20°C; log Kow: 2.34.
Discovery/Uses: Atrazine is a selective triazine herbicide used to control broadleaf and grassy weeds in corn,
sorghum, sugarcane, pineapple, christmas trees, and other crops, and in conifer reforestation plantings. It was
discovered and introduced in the late 50's. Atrazine is still widely used today because it is economical and
effectively reduces crop losses due to weed interference.
Persistence/Fate: The chemical does not adsorb strongly to soil particles and has a lengthy half-life (60 to >100
days). Atrazine has a high potential for groundwater contamination despite its moderate solubility in water.
Toxicity: The oral LD50 for atrazine is 3090 mg/kg in rats, 1750 mg/kg in mice, 750 mg/kg in rabbits, and 1000
mg/kg in hamsters. The dermal LD50 in rabbits is 7500 mg/kg and greater than 3000 mg/kg in rats. Atrazine is
practically nontoxic to birds. The LD50 is greater than 2000 mg/kg in mallard ducks. Atrazine is slightly toxic to
fish and other aquatic life. Atrazine has a low level of bioaccumulation in fish. Available data regarding
atrazine's carcinogenic potential are inconclusive.
1.3.4.1 Hexachlorocyclohexanes (HCH)
Chemical Name: 1,2,3,4,5,6-Hexachlorocyclohexane (mixed isomers) (C6H6Cl6).
CAS Number: 608-73-1 (-HCH, lindane: 58-89-9).
Properties: -HCH: solubility in water: 7 mg/L at 20°C; vapour pressure: 3.3 x 10-5 mm Hg at 20°C; log KOW:
3.8.
Discovery/Uses: There are two principle formulations: "technical HCH", which is a mixture of various isomers,
including -HCH (55-80%), -HCH (5-14%) and -HCH (8-15%), and "lindane", which is essentially pure -
HCH. Historically, lindane was one of the most widely used insecticides in the world. Its insecticidal properties
were discovered in the early 1940s. It controls a wide range of sucking and chewing insects and has been used
for seed treatment and soil application, in household biocidal products, and as textile and wood preservatives.
Persistence/Fate: Lindane and other HCH isomers are relatively persistent in soils and water, with half lives
generally greater than 1 and 2 years, respectively. HCH are much less bioaccumulative than other
organochlorines of concern because of their relatively low liphophilicity. On the contrary, their relatively high
vapor pressures, particularly of the -HCH isomer, determine their long-range transport in the atmosphere.
7
Toxicity: Lindane is moderately toxic for invertebrates and fish, with LC50 values of 20-90 µg/L. The acute
toxicity for mice and rats is moderate with LD50 values in the range of 60-250 mg/kg. Lindane resulted to have
no mutagenic potential in a number of studies but an endocrine disrupting activity.
1.3.4.2 Chlorinated Paraffins (CPs)
Chemical Name: Polychlorinated alkanes (CxH(2x-y+2)Cly). They are manufactured by chlorination of liquid n-
alkanes or paraffin wax and contain from 30 to 70% chlorine. The products are often divided in three groups
depending on chain length: short chain (C10 C13), medium (C14 C17) and long (C18 C30) chain lengths.
CAS Number: 108171-26-2
Properties: They are largely depending on the chlorine content. Solubility in water: 1.7 to 236 µg/L at 25°C;
vapour pressure: mm Hg at 20°C; log KOW: in the range from 5.06 to 8.12.
Discovery/Uses: The largest application is as a plasticiser, generally in conjunction with primary plasticisers
such as certain phthalates in flexible PVC. The chlorinated paraffins also impart a number of technical benefits,
of which the most significant is the enhancement of flame retardant properties and extreme pressure lubrication.
Persistence/Fate: CPs may be released into the environment from improperly disposed metal-working fluids or
polymers containing chlorinated paraffins. Loss of chlorinated paraffins by leaching from paints and coatings
may also contribute to environmental contamination. Short chain CPs with less than 50 % chlorine content seem
to be degraded under aerobic conditions. The medium and long chain products are degraded more slowly. CPs
are bioaccumulated and both uptake and elimination are faster for the substances with low chlorine content.
Toxicity: The acute toxicity of CPs in mammals is low with reported oral LD50 values ranging from 4 - 50 g/kg
bw, although in repeated dose experiments, effects on the liver have been seen at doses of 10 100 mg/kg
bw/day. Short-chain and mid-chain grades have been shown, in laboratory tests, to show toxic effects on fish
and other forms of aquatic life after long-term exposure. The NOEL appears to be in the range of 25 µg/L for
the most sensitive aquatic species tested.
1.3.4.3 Chlordecone
Chemical Name: 1,2,3,4,5,5,6,7,9,10,10-dodecachlorooctahydro-1,3,4-metheno-2H-cyclobuta(cd)pentalen-2-
one (C10Cl10O). Also known as Kepone.
CAS Number: 143-50-0
Properties: Solubility in water: 7.6 mg/L at 25°C; vapour pressure: less than 3*10-5 mm Hg at 25°C; log
KOW: 4.50
Discovery/Uses: Chlordecone is released to the atmosphere as a result of its manufacture and use as an
insecticide. Chlordecone also occurs as a degradation product of the insecticide Mirex. As a fungicide against
apple scab and powdery mildew former use and to control the colorado potato beetle, rust mite on non-bearing
citrus, and potato and tobacco were worm on gladisli and other plants. Chlordecone was formerly registered for
the control of rootborers on bananas Nonfood uses included wireworm control in tobacco fields and bait to
control ants and other insects in indoor and outdoor areas.
Persistence/Fate: The estimated half-life in soils is between 1-2 years, whereas in air is much higher, up to 50
years. It will not be expected to hydrolyze, biodegrade in the environment. Also direct photodegradation is not
significant similarly as evaporation from water. General population exposure to chlordecone is occurred mainly
through the consumption of contaminated fish and seafood.
Toxicity: Workers who were exposed to high levels of chlordecone over a long period (more than one year)
showed harmful effects on the nervous system, skin, liver, and male reproductive system. These workers were
probably exposed mainly through touching chlordecone, although they may have inhaled or ingested some as
well. Animal studies with chlordecone have shown effects similar to those seen in people, as well as harmful
kidney effects, developmental effects, and effects on the ability of females to reproduce. There are no studies
available on whether chlordecone is carcinogenic in people. However, studies in mice and rats have shown that
ingesting chlordecone can cause liver, adrenal gland, and kidney tumors. Very highly toxic for some species
such as Atlantic menhaden, sheepshead minnow or donaldson trout with LC50 between 21.4 56.9 mg.l-1
8
1.3.4.4 Endosulfan
Chemical Name: 6,7,8,9,10,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin-3-
oxide (C9H6Cl6O3S).
CAS Number: 115-29-7.
Properties: Solubility in water: 320 µg/L at 25°C; vapour pressure: 0.17 x 10-4 mm Hg at 25°C; log KOW: 2.23-
3.62.
Discovery/Uses: Endosulfan was first introduced in 1954. It is used as a contact and stomach insecticide and
acaricide in a great number of food and non-food crops (e.g. tea, vegetables, fruits, tobacco, cotton) and it
controls over 100 different insect pests. Endosulfan formulations are used in commercial agriculture and home
gardening and for wood preservation. The technical product contains at least 94% of two pure isomers, - and -
endosulfan.
Persistence/Fate: It is moderately persistent in the soil environment with a reported average field half-life of 50
days. The two isomers have different degradation times in soil (half-lives of 35 and 150 days for - and -
isomers, respectively, in neutral conditions). It has a moderate capacity to adsorb to soils and it is not likely to
leach to groundwater. In plants, endosulfan is rapidly broken down to the corresponding sulfate, on most fruits
and vegetables, 50% of the parent residue is lost within 3 to 7 days.
Toxicity: Endosulfan is highly to moderately toxic to bird species (Mallards: oral LD50 31 - 243 mg/kg) and it is
very toxic to aquatic organisms (96-hour LC50 rainbow trout 1.5 µg/L). It has also shown high toxicity in rats
(oral LD50: 18 - 160 mg/kg, and dermal: 78 - 359 mg/kg). Female rats appear to be 45 times more sensitive to
the lethal effects of technical-grade endosulfan than male rats. The -isomer is considered to be more toxic than
the -isomer. There is a strong evidence of its potential for endocrine disruption.
1.3.4.5 Pentachlorophenol (PCP)
Chemical Name: Pentachlorophenol (C6Cl5OH).
CAS Number: 87-86-5.
Properties: Solubility in water: 14 mg/L at 20°C; vapour pressure: 16 x 10-5 mm Hg at 20°C; log KOW: 3.32
5.86.
Discovery/Uses: It is used as insecticide (termiticide), fungicide, non-selective contact herbicide (defoliant) and,
particularly as wood preservative. It is also used in anti-fouling paints and other materials (e.g. textiles, inks,
paints, disinfectants and cleaners) as inhibitor of fermentation. Technical PCP contains trace amounts of PCDDs
and PCDFs
Persistence/Fate: The rate of photodecomposition increases with pH (t1/2 100 hr at pH 3.3 and 3.5 hr at pH 7.3).
Complete decomposition in soil suspensions takes >72 days, other authors report half-life in soils of about 45
days. Although enriched through the food chain, it is rapidly eliminated after discontinuing the exposure (t
1/2 =
10-24 h for fish).
Toxicity: It has been proved to be acutely toxic to aquatic organisms and have certain effects on human health,
at the time that exhibits off-flavour effects at very low concentrations. The 24-h LC50 values for trout were
reported as 0.2 mg/L, and chronic toxicity effects were observed at concentrations down to 3.2 µg/L.
Mammalian acute toxicity of PCP is moderate-high. LD50 oral in rat ranging from 50 to 210 mg/kg bw have
been reported. LC50 ranged from 0.093 mg/L in rainbow trout (48 h) to 0.77-0.97 mg/L for guppy (96 h) and
0.47 mg/L for fathead minnow (48 h).
1.3.4.6 Hexabromobiphenyl (HxBB)
Chemical Name: Hexabromobiphenyl (C12H4Br6).
CAS Number: 59536-65-1
Properties: Solubility in water: 11 µg/L at 25°C; vapour pressure: mm Hg at 20°C; log KOW: 6.39.
Discovery/Uses: The production of polybrominated biphebyls (PBBs) began in 1970. HxBB was used as a fire
retardant mainly in thermoplastics for constructing business machine housing and industrial (e.g. motor housing)
9
and electrical (e.g. radio and TV parts) products. Smaller amounts were used as a fire retardant in coating and
lacquers and in polyurethane foam for auto upholstery.
Persistence/Fate: HxBB is strongly adsorbed to soil and sediments and usually persist in the environment.
HxBB resists both chemical and biological degradation. HxBB has been found in several sediment samples from
the estuaries of large rivers and has been identified in edible fish.
Toxicity: Few toxicity data are available from short-term tests on aquatic organisms. The LD50 values of
commercial mixtures show a relatively low order of acute toxicity (LD50 range from > 1 to 21.5 g/kg body
weight in laboratory rodents). Oral exposure of laboratory animals to PBBs produced body weight loss, skin
disorders, and nervous system effects, and birth defects. Humans exposed through contaminated food developed
skin disorders, such as acne and hair loss. PBBs exhibit endocrine disrupting activity and possible
carcinogenicity to humans.
1.3.4.7 Polybrominated diphenyl ethers (PBDEs)
Chemical Name: Polybrominated diphenyl ethers (C12H(10-n)BrnO, where n = 1-10). As in the case of PCBs the
total number of congeners is 209, with a predominance in commercial mixtures of the tetra-, penta- and octa-
substituted isomers.
CAS Number:
Properties: Solubility in water: mg/L at 25°C; vapour pressure: 3.85 up to 13.3 x 10-3 mmHg at 20-25 °C; log
KOW: 4.28 - 9.9.
Discovery/Uses: Since the 1960s, three commercial PBDE formulations are in production. The pentabrominated
product is used principally to flame retard polyurethane foams in furniture, carpet underlay and bedding.
Commercial octa is a mixture of hexa- (10-12%), hepta- (44-46%), octa- (33-35%) and nonabromodiphenyl (10-
11%) ethers. It is used to flame retard a wide variety of thermoplastics and is recommended for injection
moulding applications such as high impact polystyrene (HIPS). The deca product (a single congener) is used
predominantly for textiles and denser plastics such as housings for a variety of electrical products in particular
TVs and computers.
Persistence/Fate: Data on environmental fate, although limited, suggest that biodegradation is not an important
degradation pathway, but that photodegradation may play a significant role. They have already been found in
high concentrations in marine birds and mammals from remote areas. The half-lives of PBDE components in rat
adipose tissue vary between 19 and 119 days, the higher values being for the higher brominated congeners.
Toxicity: The available data suggest that the lower (tetra- to hexa-) PBDE congeners are likely to be
carcinogens, endocrine disruptors, and/or neurodevelopmental toxicants. Studies in rats with commercial
PeBDE indicate a low acute toxicity via oral and dermal routes of exposure, with LD50 values > 2000 mg/kg bw.
In a 30-day study with rats, effects on the liver could be seen at a dose of 2 mg/kg bw/day, with a NOEL at
1mg/kg bw/day. The toxicity to Daphnia magna has also been investigated and and LC50 was found to be 14
µg/L with a NOEC of 4.9 µg/L. Although data on toxicology is limited, they have potential endocrine disrupting
properties, and there are concerns over the health effects of exposure.
1.3.4.8 Polycyclic Aromatic Hydrocarbons (PAHs)
Chemical Name: PAHs is a group of compounds consisting of two or more fused aromatic rings.
CAS Number:
Properties: Solubility in water: 0.00014 -2.1 mg/L at 25ºC; vapour pressure: mm Hg at 25°C; log KOW: 4.79-
8.20
Discovery/Use: Most of these are formed during incomplete combustion of organic material and the
composition of PAHs mixture varies with the source(s) and also due to selective weathering effects in the
environment.
Persistence/Fate: Persistence of the PAHs varies with their molecular weight. The low molecular weight PAHs
are most easily degraded. The reported half-lives of naphthalene, anthracene and benzo(e)pyrene in sediment are
9, 43 and 83 hours, respectively, whereas for higher molecular weight PAHs, their half-lives are up to several
10
years in soils/sediments. The BCFs in aquatic organisms frequently range between 100-2000 and it increases
with increasing molecular size. Due to their wide distribution, the environmental pollution by PAHs has aroused
global concern.
Toxicity: The acute toxicity of low PAHs is moderate with an LD50 of naphthalene and anthracene in rat of 490
and 18000 mg/kg body weight respectively, whereas the higher PAHs exhibit higher toxicity and LD50 of
benzo(a)anthracene in mice is 10mg/kg body weight. In Daphnia pulex, LC50 for naphthalene is 1.0 mg/L, for
phenanthrene 0.1 mg/L and for benzo(a)pyrene is 0.005 mg/L. The critical effect of many PAHs in mammals is
their carcinogenic potential. The metabolic actions of these substances produce intermediates that bind
covalently with cellular DNA. IARC has classified benz[a]anthracene, benzo[a]pyrene, and
dibenzo[a,h]anthracene as probable carcinogenic to humans. Benzo[b]fluoranthene and indeno[1,2,3-c,d]pyrene
were classified as possible carcinogens to humans.
1.3.4.9 Phthalates
Chemical Name: They encompass a wide family of compounds. Dimethylphthalate (DMP), diethylphthalate
(DEP), dibutylphthalate (DBP), benzylbutylphthalate (BBP), di(2-ethylhexyl)phthalate (DEHP)(C24H38O4) and
dioctylphthalate (DOP) are some of the most common.
CAS Nos.: 84-74-2 (DBP), 85-68-7 (BBP), 117-81-7 (DEHP).
Properties: The physico-chemical properties of phthalic acid esters vary greatly depending on the alcohol
moieties. Solubility in water: at 25°C; vapour pressure: mm Hg at 20°C; log KOW: 1.5 to 7.1.
Discovery/Uses: They are widely used as plasticizers, insect repellents, solvents for cellulose acetate in the
manufacture of varnishes and dopes. Vinyl plastic may contain up to 40% DEHP.
Persistence/fate: They have become ubiquitous pollutants, in marine, estuarine and freshwater sediments,
sewage sludges, soils and food. Degradation (t1/2) values generally range from 1-30 days in freshwaters.
Toxicity: The acute toxicity of phthalates is usually low: the oral LD50 for DEHP is about 25-34 g/kg,
depending on the species; for DBP reported LD50 values following oral administration to rats range from 8 to 20
g/kg body weight; in mice, values are approximately 5 to 16 g/kg body weight. In general, DEHP is not toxic for
aquatic communities at the low levels usually present. In animals, high levels of DEHP damaged the liver and
kidney and affected the ability to reproduce. There is no evidence that DEHP causes cancer in humans but they
have been reported as endocrine disrupting chemicals. The EPA proposed a Maximum Admissible
Concentration (MAC) of 6 µg/L of DEHP in drinking water.
1.3.4.10 Nonyl- and Octyl-phenols
Chemical Name: NP: C15H24O; OP: C14H22O.
CAS Number: 084852-15-3.
Properties: Solubility in water: at 25°C; vapour pressure: mm Hg at 20°C; log KOW: 4.5 (NP) and 5.92 (OP).
Discovery/Uses: NP and OP are the starting material in the synthesis of alkylphenol ethoxylates (APEs), first
used in the 60s. These compounds are highly effective cleaning agents or surfactants that have been widely used
in a number of industrial sectors including textiles, pulp and paper, paints, adhesives, resins and protective
coatings. Alkylphenols can also be used as plasticisers, stabilizers for rubbers, lube oil additives, and the
alkylphenol phosphite derivatives can be used as UV stabilisers in plastics.
Persistence/Fate: NP and OP are the end degradation products of APEs under both aerobic and anaerobic
conditions. Therefore, the major part is released to water and concentrated in sewage sludges. NPs and t-OP are
persistent in the environment with half-lives of 30-60 years in marine sediments, 1-3 weeks in estuarine waters
and 10-48 hours in the atmosphere. Due to their persistence they can bioaccumulate to a significant extent in
aquatic species. However, excretion and metabolism is rapid.
Toxicity: NP and OP have acute toxicity values for fish, invertebrates and algae ranging from 17 to 3,000 µg/L.
In chronic toxicity tests the lowest NOEC are 6 µg/L in fish and 3.7 µg/L in invertebrates. The threshold for
vitellogenin induction in fish is 10 µg/L for NP and 3 µg/L for OP (similar to the lowest NOEC). Alkylphenols
are endocrine disrupting chemicals also in mammals.
11
1.3.4.11 Perfluorooctane Sulfonate
Chemical Name: Perfluorooctane Sulfonate, C8F17SO3
CAS Number: The perfluorooctane sulfonate anion (PFOS) does not have a specific CAS number. The acid
and salts have the following CAS numbers:
acid (1763-23-1)
ammonium (NH4+) salt (29081-56-9)
diethanolamine (DEA) salt (70225-14-8)
potassium (K+) salt (2795-39-3)
lithium (Li+) salt (29457-72-5)
Properties: Solubility in water: 550 mg/l in pure water at 24-25°C; the potassium salt of PFOS has a low
vapour pressure, 3.31 x 10-4 Pa at 20°C. Due to the surface-active properties of PFOS, the Log Kow cannot be
measured.
Discovery/Uses: PFOS-related chemicals are used in a variety of products, including as surface-treatments of
fabric for soil/stain resistance, coating of paper as part of a sizing agent formulation and in specialized
applications such as fire fighting foams. The 3M Company, which started commercial production of PFOS in
1948, is the dominant producer. 3M started scaling back production in 2000. Production of PFOS by 3M is
expected to decline to zero by the end of 2002
Persistence/Fate: PFOS does not hydrolyze, photolyze or biodegrade under environmental conditions. It is
persistent in the environment and has been shown to bioconcentrate in fish. It has been detected in a number of
species of wildlife, including marine mammals. Animal studies show that PFOS is well absorbed orally and
distributes mainly in the serum and the liver. The half-life in serum is 7.5 days in adult rats and 200 days in
Cynomolgus monkeys. The half-life in humans is, on average, 8.67 years (range 2.29 21.3 years, SD = 6.12).
Toxicity: The substance shows moderate acute toxicity to aquatic organisms, the lowest LC50 for fish is a 96-
hour LC50 of 4.7 mg/l to the fathead minnow (Pimephales promelas) for the lithium salt. For aquatic
invertebrates, the lowest EC50 for freshwater species is a 48-hour EC50 of 27 mg/l for Daphnia magna and for
saltwater species, a 96-hour LC50 value of 3.6 mg/l for the Mysid shrimp (Mysidopsis_bahia). Both tests were
conducted on the potassium salt. The toxicity profile of PFOS is similar among rats and monkeys. Repeated
exposure results in hepatotoxicity and mortality; the dose-response curve is very steep for mortality. PFOS has
shown moderate acute toxicity by the oral route with a rat LD50 of 251 mg/kg. Developmental effects were also
reported in prenatal developmental toxicity studies in the rat and rabbit, although at slightly higher dose levels.
Signs of developmental toxicity in the offspring were evident at doses of 5 mg/kg/day and above in rats
administered PFOS during gestation. Significant decreases in fetal body weight and significant increases in
external and visceral anomalies, delayed ossification, and skeletal variations were observed. A NOAEL of 1
mg/kg/day and a LOAEL of 5 mg/kg/day for developmental toxicity were indicated. Studies on employees
conducted at PFOS manufacturing plants in the US and Belgium showed an increase in mortality resulting from
bladder cancer and an increased risk of neoplasms of the male reproductive system, the overall category of
cancers and benign growths, and neoplasms of the gastrointestinal tract.
1.3.5 Organometals
1.3.5.1 Organotin compounds
Chemical Name: Organotin compounds comprise mono-, di-, tri- and tetrabutyl and triphenyl tin compounds.
They conform to the following general formula (n-C4H9)nSn-X and (C6H5)3Sn-X, where X is an anion or a group
linked covalently through a hetero-atom.
CAS Number:
Properties: Solubility in water: at 25°C; vapour pressure: mm Hg at 20°C; log KOW: 3.19 - 3.84. In sea water
and under normal conditions, TBT exists as three species (hydroxide, chloride, and carbonate).
Discovery/Uses: They are mainly used as antifouling paints (tributyl and triphenyl tin) for underwater structures
and ships. Minor identified applications are as antiseptic or disinfecting agents in textiles and industrial water
systems, such as cooling tower and refrigeration water systems, wood pulp and paper mill systems, and
12
breweries. They are also used as stabilizers in plastics and as catalytic agents in soft foam production. It is also
used to control the shistosomiasis in various parts of the world.
Persistence/Fate: Under aerobic conditions, TBT takes 1 to 3 months to degrade, but in anaerobic soils may
persist for more than 2 years. Because of the low water solubility it binds strongly to suspended material and
sediments. TBT is lipophilic and tends to accumulate in aquatic organisms. Oysters exposed to very low
concentrations exhibit BCF values from 1000 to 6000.
Toxicity: TBT is moderately toxic and all breakdown products are even less toxic. Its impact on the
environment was discovered in the early 1980s in France with harmful effects in aquatic organisms, such as
shell malformations of oysters, imposex in marine snails and reduced resistance to infection (e.g. in flounder).
Molluscs react adversely to very low levels of TBT (0.06-2.3 ug/L). Lobster larvae show a nearly complete
cessation of growth at just 1.0 ug/L TBT. In laboratory tests, reproduction was inhibited when female snails
exposed to 0.05-0.003 ug/L of TBT developed male characteristics. Large doses of TBT have been shown to
damage the reproductive and central nervous systems, bone structure, and the liver bile duct of mammals.
1.3.5.2 Organomercury Compounds
Chemical Name: The main compound of concern is methyl mercury (HgCH3).
CAS Number: 22967-92-6
Properties: Solubility in water: at 25°C; vapour pressure: mm Hg at 20°C; log KOW:
Production/Uses: There are many sources of mercury release to the environment, both natural (volcanoes,
mercury deposits, and volatilization from the ocean) and human-related (coal combustion, chlorine alkali
processing, waste incineration, and metal processing). It is also used in thermometers, batteries, lamps, industrial
processes, refining, lubrication oils, and dental amalgams. Methyl mercury has no industrial uses; it is formed in
the environment from the methylation of the inorganic mercurial ion mainly by microscopic organisms in the
water and soil.
Persistence/Fate: Mercury released into the environment can either stay close to its source for long periods, or
be widely dispersed on a regional or even worldwide basis. Not only are methylated mercury compounds toxic,
but highly bioaccumulative as well. The increase in mercury as it rises in the aquatic food chain results in
relatively high levels of mercury in fish consumed by humans. Ingested elemental mercury is only 0.01%
absorbed, but methyl mercury is nearly 100% absorbed from the gastrointestinal tract. The biological half-life of
mercury is 60 days.
Toxicity: Long-term exposure to either inorganic or organic mercury can permanently damage the brain,
kidneys, and developing fetus. The most sensitive target of low level exposure to metallic and organic mercury
following short or long term exposures appears to be the nervous system.
1.3.5.3 Organolead compounds
Chemical Name: Alkyllead compounds may be confined to tetramethyllead (TML, Pb(CH3)4) and
tetraethyllead (TEL, Pb(C2H5)4).
CAS Number: 75-74-1 (TML) and 78-00-2 (TEL).
Properties: Solubility in water: 17.9 mg/L (TML) and 0.29 mg/L (TEL) at 25°C; vapour pressure: 22.5 and
0.15 mm Hg at 20°C for TML and TEL, respectively; log KOW:
Discovery/Uses: Tetramethyl and tetraethyllead are widely used as "anti-knocking" additives in gasoline. The
release of TML and TEL are drastically reduced with the introduction of unleaded gasoline in late 70's in USA
and followed by other parts of the world. However, leaded gasoline is still available which contribute to the
emission of TEL and to a less extent TML to the environment.
Persistence/Fate: Under environmental conditions such as in air or in aqueous solution, dealkylation occurs to
produce the less alkylated forms and finally to inorganic lead. However, there is limited evidence that under
some circumstances, natural methylation of lead salts may occur. Minimal bioaccumulations were observed for
TEL in shrimps (650x), mussels (120x) and plaice (130x) and for TML in shrimps (20x), mussels (170x), and
plaice (60x).
13
Toxicity: Lead and lead compounds has been found to cause cancer in the respiratory and digestive systems of
workers in lead battery and smelter plants. However, tetra-alkyllead compounds have not been sufficiently
tested for the evidence of carcinogenicity. Acute toxicity of TEL and TML are moderate in mammals and high
for aquatic biota. LD50 (rat, oral) for TEL is 35 mg Pb/kg and 108 mg Pb/kg for TML. LC50 (fish, 96hrs) for
TEL is 0.02 mg/kg and for TML is 0.11 mg/kg.
1.4 Definition of the UNEP Region of North America
The North American region, Region II, as defined by UNEP includes Canada, the United States and Mexico,
excluding the Arctic which is part of UNEP Region I, and Hawaii which is part of UNEP Region IX. Region II
is south of Region I and includes the land mass south of 60°N spanning from the Pacific Ocean to the Atlantic
Ocean. The southern boundary of Region II is the southern political border of Mexico, dividing the country from
Guatemala and Belize.
Terminology
In the text that follows, UNEP Region II North America is often referred to as simply `North America' even
though the northern part of the continent, the Arctic, is not included is this assessment report. Regions within the
North American are referred to as `sub-regions'.
1.5 Physical Setting
The North American region covers an area of approximately 16.0 x 106 km2. Its climatic zones range from
tropical wet (rainforest) and dry (savannah) in the south to sub-arctic in the north. The western portion of the
region is dominated by a number of mountain ranges, which in the south, cover most of Mexico. The central
area is a flat plain bounded on the east side by the Precambrian shield and the Appalachian Mountains. The
region is drained by seven major rivers.
The following sections describe the overall geography of the region, the ecological zones, the fresh water
systems and the climate. The last section provides a detailed description of the Great Lakes Region, an area of
special interest and concern in this assessment.
1.5.1 General Geographical Description
Canada
Canada is the world's second largest country, after Russia, and has an approximate population of 31,593,000
(July 2001 est.) with 85% of the population living within 300 km of the U.S./Canada border. The total area of
Canada is 9,976,000 km2 (with land equalling 9,221,000 km2 and water 755,000 km2) encompassing ten
provinces and three territories. This UNEP report covers approximately 5,976,000 km2 of the total area of
Canada as the Arctic portion is covered by the UNEP Region I report. Land boundaries total 8,893 km with the
U.S. being the sole border country.
Terrain in Canada consists of mostly plains with mountains in west and lowlands in southeast. The highest point
is Mount Logan at 5,959 m and the lowest point is sea level.
Natural resources in Canada consist of iron ore, nickel, zinc, copper, gold, lead, molybdenum, potash, silver,
fish, timber, wildlife, coal, petroleum, natural gas, and hydropower. Land use is estimated as follows: arable
land 5%, permanent pastures 3%, forests and woodland 54%, and other 38% (1993 est.). Irrigated land is
estimated to be 7,100 km2 (1993 est.). The GDP composition by sector in Canada is: agriculture 3%, industry
31%, and services 66% (2000 est.) (CIA 2001).
Mexico
Mexico is located in Middle America, bordering (on the east) the Caribbean Sea and the Gulf of Mexico,
between Belize and the U.S., and bordering (on the west) the North Pacific Ocean, between Guatemala and the
U.S. The population is 101,879,171 (July 2001 est.) and the total area is approximately 1,973,000 km2 (with
land equalling 1,923,000 km2 and water 50,000 km2). Land boundaries total: 4,538 km with border countries as
follows: Belize 250 km, Guatemala 962 km, and U.S. 3,326 km. The coastline is approximately 9,330 km long.
The terrain consists of high, rugged mountains, low coastal plains, high plateaus and desert. The highest point is
Volcan Pico de Orizaba at 5,700 m and the lowest point is Laguna Salada at -10 m.
14
Natural resources in Mexico consist of petroleum, silver, copper, gold, lead, zinc, natural gas, and timber. Land
use is as follows: arable land 12%, permanent crops: 1%, permanent pastures 39%, forests and woodland 26%,
and other 22% (1993 est.). Irrigated land is approximately 61,000 sq km (1993 est.). The GDP composition by
sector in Mexico is as follows: agriculture 5%, industry 27%, and services 68% (2000 est.) (CIA 2001).
Natural hazards in Mexico consist of tsunamis along the Pacific coast, volcanoes and earthquakes in the centre
and south, and hurricanes on the Gulf of Mexico and Caribbean coasts.
The United States of America
The United States of America is the world's fourth-largest country (after Russia, Canada, and China) and has an
approximate population of 278,059,000 (July 2001 est.) The area of the United States is approximately
9,629,000 km2 (with land area equalling 9,159,000 km2 and water equalling 470,000 km2) encompassing 50
states. This report covers approximately 8,000,000 km2 of the total area of the United States as the Arctic
portion, Alaska, and Hawaii are covered elsewhere.
Land boundaries with border countries total 12,248 km and are as follows: Canada 8,893 km (including 2,477
km with Alaska), Cuba 29 km (U.S. Naval Base at Guantanamo Bay), and Mexico 3,326 km.
The terrain of the United States consists of a vast central plain, mountains in the west, hills and low mountains
in the east. The highest point is on Mount McKinley at 6,194 m and the lowest point is Death Valley at -86 m.
Land use in the United States is estimated as follows: arable land 19%, meadows and pastures 25%, forest and
woodland 30%, other 26%. Irrigated land is estimated to be 207,000 km2 (1993 est.) (CIA 2001).
Natural resources in the U.S. consist of: coal, copper, lead, molybdenum, phosphates, uranium, bauxite, gold,
iron, mercury, nickel, potash, silver, tungsten, zinc, petroleum, natural gas, and timber. The GDP composition
by sector in the United States is: agriculture 2%, industry 18%, and services 80% (1999 est.) (CIA 2001).
In the United States, desertification is an issue as are natural hazards such as: tsunamis, volcanoes, and
earthquake activity around Pacific Basin; hurricanes along the Atlantic coast; tornadoes in the Midwest; mud
slides in California; forest fires in the west; and flooding.
1.5.2 Ecological Zones
North America is a continent rich in diversity. Climatic types range from the polar arctic to tropical forests.
Topographically, the continent contains a valley with the lowest elevation on earth and also extensive chains of
tall mountains. It is rich in natural resources and scenic natural beauty. North America has great variety in its
populations of native animals and plants, since before recorded history, the development of a rich diversity in
human cultures has occurred.
The CEC report "Ecological Regions of North America" (CEC 1997) divides the continent into 15 broad,
ecological regions as shown in the Figure 1.
15
Figure 1: Ecological Regions of North America
This map provides a broad description of the ecology of the continent. The ecological regions are: Arctic
Cordillera, Tundra, Taiga, Hudson Plains, Northern Forests, Northwestern Forested Mountains, Marine West
Coast Forests, Eastern Temperate Forests, Great Plains, North American Deserts, Mediterranean California,
Southern Semi-Arid Highlands, Temperate Sierras, Tropical Dry Forests and Tropical Humid Forests
(CEC1997).
The first three regions (Arctic Cordillera, Tundra, and Taiga) lie entirely in the Arctic and are covered by the
UNEP Region I report. The other 12 regions are described below.
The Hudson Plains
The Hudson Plains ecological region, lying entirely in Canada, is centred in northern Ontario and extends into
north-eastern Manitoba and western Quebec. Wetlands cover 90 percent of this ecological region, making it the
largest wetland-dominated area of North America. This region contains the longest stretch of shallow, emergent
wetland shoreline on Earth. The population of 10,000 is largely aboriginal. Hunting, fishing and trapping with
some tourism are the major activities (CEC 1997).
The Northern Forests
This ecological region is broad and crescent-shaped, extending from northern Saskatchewan east to
Newfoundland and south to Pennsylvania, lying to the north of the Eastern Temperate Forests region. It is
distinguished by extensive boreal forests and a high density of lakes situated on the Canadian Shield. Despite
having many urban areas, highways, railways, roads and airports, much of this ecological region remains a
16
relative wilderness. With a population of 4 million, this is a core area for forest and mining activities.
Commercial fishing is extensive on its east coast (CEC 1997).
Northwestern Forested Mountains
This ecological region extends southward from the northern border of Region II (60°N), through interior British
Columbia and the Alberta foothills, through northern California and over into Nevada. It contains the highest
mountains of North America and some of the continent's most diverse mosaics of ecosystem types, ranging
from alpine tundra to dense conifer forests to dry sagebrush and grasslands. There are major river systems,
including the headwaters to both the Fraser and Columbia rivers. The basis for aggregating all this diversity into
one ecological region is topographic --the chains of mountains that traverse its whole length. This region of
800,000 people is a major tourist area for skiing, hiking and other outdoor recreational pursuits. Substantial
forestry and mining activity occur throughout (CEC 1997).
Marine West Coast Forests
This ecological region covers the mainland and offshore islands of the Pacific Coast from the north-western
boundary of Region II (60°N) south to northern California. The wettest climates of North America occur in this
area. It is characterized by mountainous topography bordered by coastal plains, and contains all of the temperate
rain forests found in North America. These forests are among the most productive in North America, making
forestry the major resource activity. Major commercial fisheries occur offshore. The large population of 6.5
million is concentrated in coastal cities and towns (CEC 1997).
Eastern Temperate Forests
This ecological region extends from the Great Lakes in the north to the Gulf of Mexico in the south. From the
Atlantic Coast, it extends westward approximately 600 km into eastern Texas, Oklahoma, Missouri, Iowa and
Minnesota. The region is distinguished by its moderate to mildly humid climate, its relatively dense and diverse
forest cover, and its large population of approximately 160 million. Urban industries, agriculture and some
forestry are major activities (CEC 1997).
Great Plains
The Great Plains ecological region is found in the central part of the continent and extends over the widest
latitudinal range of any single North American ecological region. It is a relatively continuous and roughly
triangular area covering about 3.5 million square kilometers. The prairies extend from Alberta, Saskatchewan
and Manitoba in Canada, south through the Great Plains of the United States to southern Texas and adjacent
Mexico, and from western Indiana to the foothills of the Rockies and into north-eastern Mexico. This region is
distinguished by the following characteristics: relatively little topographic relief; grasslands and a paucity of
forests; and sub humid to semiarid climate (CEC 1997).
North American Deserts
This ecological region extends from south-eastern British Columbia in the north, to Baja California and north
central Mexico in the south. The region is distinguished from the adjacent forested mountain ecological region
by its aridity, its unique shrub and cactus vegetation with a lack of trees, and generally lower relief and
elevations. Population centres have historically been small, but several urban areas like Las Vegas have recently
experienced rapid growth (CEC 1997).
Mediterranean California
This relatively small ecological region is contained entirely in the United States and extends 1,300 km from
Oregon in the north to Baja California Norte state in the south. It abuts the Pacific Ocean on the west and the
Sierra Nevada and deserts to the east. It is distinguished by its warm and mild Mediterranean climate, its
shrubland vegetation of chaparral mixed with areas of grassland and open oak woodlands, its agriculturally
productive valleys and its high population (30 million) in extensive urban agglomerations (CEC 1997).
Southern Semi-Arid Highlands
This region extends over part of the states of Arizona and New Mexico in the United States, and southward over
several states in northern, western and central Mexico. In Mexico, this region is bounded on the west by the
Temperate Sierras and on the east by the North American Deserts ecological region. The landscape is composed
17
of hills, bottom valleys and plains. In general, the vegetation within this region is dominated by grasslands and
in the transition zones by various scrublands and forests (CEC 1997).
Temperate Sierras
This ecological region comprises the major Mexican mountains including the Western Sierra Madre, the Eastern
Sierra Madre, the Nudo Mexteco in western Oaxaca and Chiapas. Overall, the region covers approximately 25
percent of the land area of Mexico. Many of the major cities of the country are located here, including Mexico,
Guadalajara, Morelia, Toluca and Puebla. Approximately 40 million people inhabit this region of intensive
agricultural and industrial use (CEC 1997).
Tropical Dry Forests
This ecological region stretches in a narrow and interrupted strip from Eastern Sonora and South-eastern
Chihuahua to Chiapas; at Michoacán it includes the Balsas Basin. In the Tehuantepec isthmus, it splits to
include the Central Chiapas Depression where it stretches along the Pacific to Central America and the northern
extreme of South America. It also occupies the Northern Gulf Coastal Plain, the north of the Yucatán Peninsula
and the southern tip of the Baja California Peninsula, covering almost 13 percent of Mexico (CEC 1997).
Tropical Humid Forests
This ecological region includes the southern tip of the Florida Peninsula in the United States. Within Mexico, it
encompasses the Gulf Coastal Plain, the western and southern part of the Pacific Coastal Plain, most of the
Yucatán Peninsula and the lowlands of the Chiapas Sierra Madre, which continue south to Central and South
America. Approximately 20.4 million inhabitants live in this ecological region. Of this, over 16 million live in
the Mexican portion, an area that has seen a 30 percent increase in population since 1980. The greatest numbers
of indigenous peoples who are descendants of the great cultures, such as the Maya, live in this region (CEC
1997).
1.5.3 Freshwater
There are very limited natural fresh water resources in much of the south-western United States. The same
situation prevails in the south-central plain area of Canada. In the north-central and north-eastern portion of the
region, there are innumerable lakes, estimated to run into the millions. These lakes are relics of past glaciation.
In Mexico, natural fresh water resources are scarce and of poor quality in the north, inaccessible and of poor
quality in the centre and extreme southeast.
North America is drained by a number of major rivers. Seven have been selected here for detailed description
(Table 1). The choice of these rivers is based on their length, their drainage area and their mean flow rates. The
river with the largest drainage area is the Mackenzie River at 1,805,200 km2. A significant portion of this
drainage area lies in UNEP Region I. The St. Lawrence River has the largest annual discharge rate at 9,850 m3/s.
Table 1: Characteristic of seven of the major river basins in UNEP REGION II, North America
RIVER SYSTEM
LENGTH
DRAINAGE AREA
DISCHARGE AT MOUTH
(km to source of
(1 000 sq. km)
(cu. meters per second)
longest tributary)
Mississippi- -Missouri
6 019
Can.: 28
17 545
US: 3 220
Total: 3 248
Mackenzie
4 250
1 805
9 700
Saint Lawrence
3 100
Can.: 839
9 850
US: 505
Total: 1 344
Rio Grande
2 870
570
82
(Río Bravo del Norte)
Colorado 3
200
629
168
18
RIVER SYSTEM
LENGTH
DRAINAGE AREA
DISCHARGE AT MOUTH
(km to source of
(1 000 sq. km)
(cu. meters per second)
longest tributary)
Columbia
2 250
Can.: 103
7 770
US: 568
Total: 671
Nelson
2 575
Can.: 803
2 633
US: 180
Total: 983
The total drainage area for these seven rivers is 10.9 x 106. This is nearly 70 percent of the total area of UNEP
Region II. Two of the rivers, the Rio Grande and the Colorado, flow through the arid area of central southern
U.S.A. The lack of annual precipitation, high evaporation rates, and extensive use of river water for irrigation,
have led to very low discharge rates when compared to the other rivers in this group. The information in Table 1
has been derived from several sources (US Army Corps of Engineers 1997, River Systems of the World and
National Atlas of Canada 1985). In general, the sources are in fairly close agreement with one another.
Differences may be due to the manner in which the length of the river is determined and the way in which the
seasonal flow rates are measured to obtain the annual average value.
1.5.4 Climate and Meteorology
The climate in the UNEP Region - North America ranges from sub-arctic, to dry and humid continental, to
tropical in the south. (Trewartha 1954)
Canada
The Canadian climate varies from temperate in the south to sub-arctic and arctic in north.
Cyclonic storms form east of the Rocky Mountains, a result of the mixing of air masses from the Arctic, Pacific,
and North American interior. These storms produce most of Canada's rain and snow.
Dramatic temperature changes mark Canada's seasons. The interior has a continental climate, colder than
coastal regions in winter, warmer in summer. Only south-western British Columbia is temperate; temperatures
here rarely fall below freezing (Atlas of Canada 1981).
The Rockies protect the west coast from the Arctic's air masses, but on their east side, they also funnel the cold
northern air toward the south and east, making Prairie winters long and bitterly cold. Snow cover sends
temperatures lower by reflecting great amounts of sunshine. For their latitudes, southern Ontario and Quebec
rank among the earth's coldest places. Even so, cactuses grow on Canada's southernmost tip, Point Pelee in
Ontario (Atlas of Canada 1981).
Snow accumulation reaches the seasonal maximum in February over Canada. The area around the Great Lakes
can experience extreme blizzards, but the north-west coast is noted for mild, wet winters. East of The Rockies,
the "Chinook" wind can raise temperatures by up to 22 °C (40 °F) in an hour or so. Average daytime
temperatures vary from 7 °C (45 °F) at Vancouver, to -1 °C (30 °F) at Toronto. The west coast is wet, with
about 10 dry days each month on average, while Toronto averages 16-17 such days (Met Office 2002).
The transition from winter to summer occurs very quickly. From mid-April, within a few weeks, the daily
average temperature at Toronto rises from 4 °C (39 °F) to 18 °C (66 °F). In March, the average number of dry
days ranges from 14 in the west to 19 at Toronto (Met Office 2002).
In much of the southern half of Canada, the summer is warm (25 °C / 77 °F) and sunny with only moderate
rainfall (about 50-100 mm per month). Sea fog is sometimes rather persistent on the east coast (Met Office
2002).
19
There is a marked decrease in daily maximum temperatures during the autumn months, in Toronto dropping
from 22 °C (70 °F) in September, to 7 °C (45 °F) in November. Dry days vary from 16-21, although Vancouver
has a wetter November (12 days) with a daily average of about three sunshine hours (Met Office 2002).
Mexico
The Mexican climate varies from tropical to desert. Almost two-thirds of Mexico consists of plateaux and high
mountains with a climate that is warm-temperate; other parts have a tropical climate.
There are three important influences which help to determine the character of the climate of different parts of
Mexico. The cold Californian current which sweeps southwards on the Pacific coast has the effect of lowering
temperatures and reducing rainfall on the west coast as far south as the tip of the peninsula of Lower California.
This and the influence of the North Pacific anticyclone help to make much of the northwest of the country desert
or semi-desert; this is a continuation of the dry zone of the U.S.A. in California, New Mexico and Arizona (Met
Office 2002).
The warm Caribbean Sea and the influence of the constant north-east trade winds make the eastern coastal
region a typical tropical coast with a marked single wet season in summer (Met Office 2002).
The third important influence is that of the climate of Canada and the United States to the north. This area
becomes very cold in winter, particularly when cold air sweeps down from the Canadian Arctic, and also very
warm in summer. Northern Mexico shares in these extreme temperature conditions. In winter, cold waves
('northers') can bring near-freezing conditions for a few days to the east coast as far south as Tampico. Snow has
fallen at Tampico which lies within the tropics. The west coast is protected from such cold waves by the
mountains and plateaux of central Mexico (Met Office 2002).
As in other South and Central American countries, the climatic zones are described on the basis of altitude:
tierra caliente (below 2000 feet); tierra templada (2000 - 6000 feet); tierra fria (above 6000 feet). Only a very
narrow coastal belt on the Pacific coast falls into the tierra caliente category, but there is a more extensive area
on the Caribbean shore, including the whole Yucatan peninsula. The largest part of Mexico falls into the other
two categories. In most of the terra fria, frost is frequent at night in winter and snow can occur anywhere, but
only lies above 1000 - 12000 feet (Met Office 2002).
The rainy season over the whole country is the period of high sun from May to October, and the wettest part of
the country is the lowland on the Caribbean coast. Annual rainfall in these parts is between 1000 and 1500 mm,
but some places in northern Yucatan get less than 500 mm. The more northern shores of the Pacific and Gulf of
California receive less than 250 mm per year, but this increases southwards to between 1000 and 1500 mm.
Where the coast is backed by high mountains rainfall is heaviest (Met Office 2002).
Most of Mexico has sunny weather for a large part of the year. The cloudiest regions are the wetter parts of the
east coast and the northern part of the Pacific coast where fog and low cloud are formed over the cold ocean
current. The drier regions of the interior and much of the tierra templada have high sunshine amounts, as much
as eight hours a day in the drier months (Met Office 2002).
The United States of America
The climate of the U.S. is mostly temperate, but tropical in Florida, semiarid in the Great Plains west of the
Mississippi River and arid in the Great Basin of the southwest. Low winter temperatures in the northwest are
ameliorated occasionally in January and February by warm Chinook winds from the eastern slopes of the Rocky
Mountains (CIA 2001).
1.5.5 The Great Lakes sub-region
The Great Lakes Basin covers an area roughly 774,000 square kilometres including almost all of southern
Ontario, parts of northern Ontario and the eight Great Lakes States: Illinois, Indiana, Michigan, Minnesota, New
York, Ohio, Pennsylvania and Wisconsin. For comparison, this area is over twice the size of Germany or nearly
40% of the size of Mexico. If the St. Lawrence River drainage basin is included, the area becomes 1,344,000
square kilometres (Table 1).
20
The Great Lakes (Superior, Michigan, Huron, Erie and Ontario) span more than 1,200 kilometres (750 miles)
from west to east, and are home to a total of about 33 million people, more than one-tenth of the population of
the United States and one-quarter of the population of Canada. Over thirty million people in the U.S. and in
Canada rely on the Great Lakes watershed as a source of drinking water (Government of Canada & US EPA
1995).
The lakes contain about 23,000 km3 (5,500 cu. mi.) of water, covering a total area of 244,000 km2 (94,000 sq.
mi.). The Great Lakes are the largest system of fresh, surface water on earth, containing roughly 18 percent of
the world freshwater resources. Outflows from the Great Lakes are relatively small (less than 1 percent per year)
in comparison with the total volume of water (Government of Canada & U.S. EPA 1995).
The wealth of natural resources has long made the region a heartland of both the U.S. and Canadian industrial
economy. Some of the world's largest concentrations of industrial capacity are located in the Great Lakes region.
Agriculture in Ontario and Quebec accounts for the largest single use of Canadian land in the basin and
contributes about 40 percent of the value of agricultural output in the Canadian economy, helping to feed the
more than 16 million consumers in the region (CESD 2001). Nearly 7 percent of the American agricultural
production is located in the basin (Government of Canada & U.S. EPA 1995). The region generates more than
50 percent of the total U.S. manufacturing output. Economic activity in the Great Lakes basin exceeds $200
billion a year. The international shipping trade annually transports 50 million tons of cargo through the Great
Lakes. The annual value of the commercial and sport fishery is estimated at over $4.5 billion (U.S. EPA 2002).
Lake Superior is the largest of the Great Lakes. Superior could easily contain all the other Great Lakes. Because
of its size, Superior has a retention time of 191 years (Government of Canada & U.S. EPA 1995).
Lake Michigan, the second largest, is the only Great Lake entirely within the United States. The more temperate
southern basin of Lake Michigan is among the most urbanized areas in the Great Lakes system. It contains the
Milwaukee and Chicago metropolitan areas. This region is home to about 8 million people or about one-fifth of
the total population of the Great Lakes basin (Government of Canada & U.S. EPA 1995).
Lake Huron, which includes Georgian Bay, is the third largest of the lakes by volume. Many Canadians and
Americans own cottages on the shallow, sandy beaches of Huron and along the rocky shores of Georgian Bay
(Government of Canada & U.S. EPA 1995).
Lake Erie is the smallest of the lakes in volume and is exposed to the greatest effects from urbanization and
agriculture. Because of the fertile soils surrounding the lake, the area is intensively farmed. Although the area of
the lake is about 26,000 km2 (10,000 square miles), the average depth is only about 19 metres (62 feet). Lake
Erie has the shortest retention time of the lakes at 2.6 years (Government of Canada & U.S. EPA 1995).
Lake Ontario, although slightly smaller in area, is much deeper than Lake Erie, with an average depth of 86
metres (283 feet) and a retention time of about 6 years. Major urban industrial centers, such as Hamilton and
Toronto, are located on its shore. The U.S. shore is less urbanized and is not intensively farmed, except for a
narrow band along the lake (Government of Canada & U.S. EPA 1995).
1.6 References
Atlas of Canada, 1981.The Reader's Digest Association (Canada) Ltd.
Blankenship, A.L., Kannan, K., Villalobos, S.A., Vileeneuve, D.L., Falandysz J., Imagawa, T., Jakobsson E. and
Geisy, J.P., 2000. Relative Potencies of Individual Polychlorinated Naphthalenes and Halowax Mixtures to
Induce Ah Receptor-mediated Responses, Environ. Sci. Technol., 34, 3153.
Berg, M.V., Birnbaum, L., Bosveld A.T.C., Brunstrom, B., Cook, P., Feeley, M., Giesy, J.P., Hanberg, A.,
Hasegawa, R., Kennedy, S.W., Kubiak, T., Larsen, J.C., Leeuwen, F.X.R., Liem, A.K.D., Nolt, C., Peterson,
R.E., Poellinger, L., Safe, S., Schrenk, D., Tillitt, D., Tysklind, M., Younes, M., Waern, F. and Zacharewski, T.
1998. Toxic Equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for Humans and Wildlife, Environmental
Health Perspectives, 106, 775.
21
CEC (Commission for Environmental Cooperation), 1997. Ecological Regions of North America Toward a
Common Perspective.
CESD 2001. Commissioner of the Environment and Sustainable Development, 2001 Report, Ottawa.
CIA (Central Intelligence Agency) 2001. The World Factbook 2001.
http://www.odci.gov/cia/publications/factbook/index.html
Government of Canada and United States Environmental Protection Agency (USEPA), 1995. The Great Lakes,
An Environmental Atlas and Resource Book. http://www.epa.gov/glnpo/atlas/
Met Office 2002. United Kingdom government. http://www.met-office.gov.uk
National Atlas of Canada, 1985. 5th ed.: Drainage Basins: Energy, Mines and Resources Canada and
Environment Canada, Ottawa. http://atlas.gc.ca/site/english/facts/rivers.html#hudson
River Systems of the World. Provided by: Rev.Net Technologies, Inc., Roanoke, VA, USA.
http://www.rev.net/~aloe/river/
Trewartha, Glenn T., 1954. An Introduction to Climate. McGraw-Hill Book Company, Inc., Toronto.
US Army Corps of Engineers (1997). The Columbia-Snake River Basin. http://www.nwd-
wc.usace.army.mil/TMT/basin.html
22
2. SOURCE CHARACTERIZATION
2.1. Background Information on PTS Sources
2.1.1 Data Management in Canada and the USA
The primary U.S. database used in this subsection is the U.S. EPA Toxics Release Inventory (TRI), which began
collecting information for the year 1987. The comparable Canadian database used in this report is Environment
Canada's National Pollutant Release Inventory (NPRI) started in 1993. The two inventories have many basic
similarities since they stem from the same primary purpose--to provide publicly available information on
releases and transfers to air, water and land through a mandatory reporting mechanism (CEC 2002).
The original TRI list contained over 300 chemicals, covered manufacturing sectors, and required information on
on-site releases, transfers off-site for disposal and transfers offsite for treatment. Passage of the Pollution
Prevention Act of 1990 broadened the information TRI collects to include off-site transfers to recycling and
energy recovery as well as facilities' management of toxic chemicals in waste on-site, such as on-site treatment,
recycling and energy recovery, as well as qualitative information on pollution prevention activities at the
facility. There are now over 650 chemicals on the TRI list, including the 12 chemicals listed in the Stockholm
Convention on Persistent Organic Pollutants (CEC 2002; UNEP 2001).
Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA), the law that created
TRI, had identified the manufacturing sector as the original set of industries required to submit TRI reports.
Beginning with the 1998 reporting year, several new industries were added to TRI. The seven new industrial
sectors added were metal mines, coal mines, electricity-generating facilities, petroleum bulk storage terminals,
chemical wholesale distributors, hazardous waste management facilities and solvent recovery facilities (CEC
2002).
NPRI requires information on on-site releases and off-site transfers to treatment and disposal. Starting with the
1997 reporting year, Environment Canada required mandatory reporting on pollution prevention activities. Off-
site transfers to recycling and energy recovery were made mandatory, beginning with the 1998 reporting year
(CEC 2002, Environment Canada 2002b).
Currently, there are over 270 substances listed on the NPRI. Some important changes beginning for the 2002
reporting year, include the addition of criteria air contaminants (CACs, the thresholds have been lowered for
several heavy metals, and reporting of pollution prevention activities has been expanded). The inventory has
also expanded to capture reporting from smaller facilities, such as incinerators, wood preservers, and wastewater
collection and treatment systems.
2.1.2 Data Management in Mexico
2.1.2.1 Mexican Source Profile
In Mexico, the increasing development of the industrial sector is associated with the production and use of a
wide variety of chemical substances and compounds, such as pesticides, fertilizers, additives, food and beverage
preservatives, resins, synthetic fibers, plastics, fuels, solvents, etc. The use of these materials has led to the
deterioration of environmental quality by their derivatives and wastes, heightening the risk to the health and
physical integrity of the general populace.
In this sense, the need arises to apply comprehensive regional solutions to standardize procedures, criteria,
actions and control programs, to effectively mitigate and reduce the high level of accidents involving a wide
range of chemicals and the chronic exposure to toxic materials by the community in general.
The Mexican manufacturing industry sector is classified under Division 3 by the National Institute of Statistics,
Geography and Information (Instituto Nacional de Estadística, Geografía e Informática--Inegi) and includes 11
categories, one of which is "Chemicals and Oil Derivatives, Rubber and Plastic," which in turn comprises
several areas or activities involving a variety of chemical substances. In general terms, this category represents
18% of the manufacturing industry division, which accounts for 22.5% of the Gross Domestic Product (GDP).
23
The percentage distribution of the GDP corresponding to the chemicals sector, broken down by area, is set forth
in Figure 2
Breakdown of Gross Domestic Product (GDP)
by Chemical Sector
Basic Petrochemicals
Basic Chemicals
Plastics
9.80%
9.80%
13.60%
Rubber Products
Manure and Fertilizers
9.90%
1.60%
Other Chemical
Products
Synthetic Resins and
14%
Artificial Fibers
Soaps, Detergents and
Pharmaceuticals
13%
Cosmetics
13.20%
15.00%
Figure 2: Mexico: Break down of Gross Domestic Product by Chemical Sector
This category covers all chemical substances imported, produced, sold and applied in national territory. In this
regard, according to data from the National Association of the Chemical Industry (Asociación Nacional de la
Industria Química--ANIQ), there are 500 listed industrial facilities, including the petrochemical plants of
Petróleos Mexicanos (PEMEX), which are located throughout Mexico as shown in Figure 3.
Distribution of
Chemical Industry Plants
Tlaxcala
Tamaulipas
Hidalgo
2% Nuevo León
Mexico State
5% Guanajuato
2%
7%
26%
5%
Jalisco
5%
Chapas,
Oaxaca and
Federal District
Tabasco
16%
2%
Durango, Chuhuahua,
Queretaro,
Veracruz
San Luis Potosí
Morelos Puebla
Coahuila and
16%
and Michoacán
3%
4%
Baja California
4%
3%
Figure 3: Mexico: Distribution of Chemical Industry Plants
24
The activities of the chemicals sector with the greatest use of persistent toxic substances (PTSs) are described
below:
Petrochemical Industry
The activities of PEMEX-Petroquímica in Mexico involve a series of industrial processes, the products of which
form part of the basic petrochemicals industry, as well as the storage, distribution and sale thereof.
· The commercial products deemed basic are ethane, propane, butane, pentane, hexane, heptane, raw
materials for lamp-black, and naphtha.
· Secondary petrochemicals, the manufacture of which requires a permit, are acetylene, ammonia, benzene,
butadiene, butylene, ethylene, n-paraffins, orthoxylene, paraxylene, propylene, toluene and xylene.
Agrochemical Industry
This industry is responsible for the production of two major groups: fertilizers and pesticides.
· Fertilizers and chemical products containing principally nitrogen, phosphorus or potassium. Their primary
function is to act as a source of plant nutrients.
· Pesticides are those substances or mixtures intended to destroy, control, prevent or repel the action of any
form of harmful animal or plant life. Some characteristic substances in this group are insecticides, desiccants
and defoliants.
The production and sale of these substances in Mexico was traditionally carried on through Fertilizantes
Mexicanos, S.A. (FERTIMEX), a government-owned entity that, after operating for more than 30 years, began a
plant sell-off process in 1991. The first plant, the Torreón plant, was sold on 4 March 1991, and the process
concluded on 21 December 1992 with the sale of the Lázaro Cárdenas plant.
The fertilizers most used in Mexico are nitrogenates and phosphates, while the pesticides most commonly used
by the agricultural sector have traditionally been DDT, methyl parathion and Servín, as well as acid herbicide
2,4-D.
2.1.2.2 Data compilation and quality control in Mexico
The compilation and assessment of data relating to the different stages of PTS handling was not an easy task,
due to the variety of public-sector entities having jurisdiction over such handling pursuant to the official
institutional framework.
In this regard, it is appropriate to specify the participation of the government agencies involved in the handling
of PTSs, given that the information used in developing this report was obtained from them.
The actions of the Secretariat of Health (Secretaría de Salud--SSA) are based on two bodies of rules governing
chemical substances and products, namely the General Health Law (Ley General de Salud--LGS) and the
Regulations to the General Health Law Regarding the Health Control of Activities, Establishments, Products
and Services (Reglamento de la Ley General de Salud en Materia de Control Sanitario de Actividades,
Establecimientos, Productos y Servicios--RCS). The LGS and RCS together provide the definitions of
pesticides, fertilizers and toxic substances. They establish a registration, licensing and permit regime for such
products and also provide the standards for chemical labelling.
Along with other related authorities, the LGS empowers the SSA to establish the Mexican Official Standards
(Normas Oficiales Mexicanas--NOMs) to be observed with respect to the use, application, development,
nomenclature, storage, commercialization and distribution of all chemical substances.
The LGS also provides that the SSA must oversee the granting of permits relating to toxic substances, pesticides
and fertilizers not intended for agricultural or forestry uses. Other laws also govern chemical substances.
In environmental matters, the Secretariat of Environment and Natural Resources (Secretaría del Medio
Ambiente y Recursos Naturales--Semarnat) acts in accordance with the General Law of Ecological Balance and
Environmental Protection (Ley General del Equilibrio Ecológico y la Protección del Ambiente--Lgeepa), which
25
establishes general rules applicable to chemicals as part of its provisions on the control and prevention of soil
pollution. The Lgeepa generally requires that the use of pesticides, fertilizers and toxic substances be compatible
with the ecological balance of the ecosystems.
The Lgeepa also sets the bases for the cleanup and recovery contaminated soils for reuse. The Law empowers
Semarnat to develop, along with the Secretariat of the Economy (Secretaría de Economía--SE) and the
Secretariat of Agriculture, Stockbreeding, Rural Development, Fisheries and Nutrition (Secretaría de
Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación--Sagarpa) and the SSA, the regulations and
NOMs for pollution prevention and control with respect to chemical substances.
Furthermore, Lgeepa Article 144 prohibits the importation of pesticides, fertilizers and other hazardous
substances when the usage thereof is not permitted in the country of origin.
The Federal Law on Animal Health (Ley Federal de Sanidad Animal--LFSA), as it relates to phytosanitary and
feeding aspects, provides Sagarpa with the authority to issue NOMs on animal products and by-products,
including biological, chemical, pharmaceutical and feed products used or consumed by animals. Sagarpa also is
responsible for the registration and authorization and pesticides and fertilizers intended for agricultural or
forestry uses.
The Federal Law on Plant Health (Ley Federal de Sanidad Vegetal--LFSV) gives Sagarpa the authority to
develop standards for phytosanitary activities relating to the production, industrialization, transport or
distribution of plants, plant products or materials used in plant production, including fertilizers and pesticides.
The Federal Consumer Protection Law (Ley Federal de Protección al Consumidor) establishes rules for the
advertising, labelling, packaging, quality assurance, safety and warranties for chemical products and services in
general. These rules are administered and enforced by the SE and the Office of the Federal Attorney General for
Consumer Affairs (Procuraduría Federal del Consumidor).
In addition, the Federal Labor Law (Ley Federal del Trabajo--LFT) and the Workplace Safety and Hygiene
Regulations (Reglamento de Seguridad e Higiene en el Lugar de Trabajo--RSH) empower the Secretariat of
Labour and Social Welfare (Secretaría del Trabajo y Previsión Social--STPS) to establish worker protection
standards in workplaces where chemical substances are an integral part of the business operations or where they
may pose an occupational health risk. The STPS and the SSA both have jurisdiction over the application of
worker protection standards.
The involvement of the Secretariat of Communications and Transportation (Secretaría de Comunicaciones y
Transportes--SCT) is based on the Regulation on Ground Transportation of Hazardous Materials and Waste
(Reglamento para el Transporte Terrestre de Materiales y Residuos Peligrosos), which includes chemical
substances.
To coordinate the actions and enforcement relating to toxic substances in general, a presidential decree
published on 15 October 1987 created the Intersecretarial Commission for the Control of Pesticides, Fertilizers
and Toxic Substances (Comisión Intersecretarial para el Control de Plaguicidas, Fertilizantes y Sustancias
Tóxicas--CICOPLAFEST). This commission, composed of representatives from the SSA, Semarnat, SE and
Sagarpa, has a Chairman, a Technical Committee with its coordinator, and five subcommittees and their
respective coordinators. The CICOPLAFEST Internal Regulations state that each Secretariat shall be
represented on each subcommittee. At present, no more than 20 technical advisers from all Secretariats
participate, as compared to countries like the United States and Canada, which respectively have 400 and 250
technical specialists to carry on activities solely related to pesticide regulation.
CICOPLAFEST has the attributions indicated in the Bases for Coordination and the Internal Regulations, which
set forth the uniform and comprehensive procedures for resolving on registration and authorization requests for
pesticides, fertilizers and toxic substances, with regard to the use, preparation, manufacture, formulation,
mixing, conditioning, packaging, handling, transport, distribution, application, storage, commercialization,
possession and final disposal thereof. For this purpose, CICOPLAFEST has a Single Window to attend to any
request regarding the aforesaid procedures.
26
Though the systems for the tracking of the production, use and emissions of PTSs is progressing rapidly in
Mexico, at this time the data management infrastructure is incomplete and hence it is not possible to portray the
Mexican information one chemical at a time. Overall, it is estimated that about 150 pesticides are in use,
including some which are considered banned, such as the HCB (on 13 States), ethylic parathion (on 15 States)
and toxaphene (on 2 States). Tamaulipas, Chiapas, Hidalgo y Sonora, are the States where a high quantity of
banned products are employed.
2.2 Production, Use and Emissions
Information on production or use of pesticides is often proprietary, and quantitative estimates of production of
can be difficult to obtain. Chemical manufacturers in the United States however, can legally produce pesticides
for export that are currently banned or not registered for use in the United States (ATSDR 2002).
In the following discussion, the unit of weight in the U.S. data is usually pounds while the Mexican and
Canadian unit of weight is tonnes. The conversions are: one ton equals 2000 pounds and one tonne (1000 kg)
equals 2,204.6 pounds.
2.2.1 Pesticides
2.2.1.1 Aldrin and Dieldrin
Aldrin is readily converted to dieldrin in the environment, and thus these two substances are described together.
Source: Aldrin was first synthesized in the United States as a pesticide in 1948. It was used as an insecticide
from the 1950s to early 1970s on cotton and corn crops. In 1974, all uses except termite control were cancelled
under the U.S. Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), and production in the U.S. ceased.
Aldrin has not been imported since 1985 due to health concerns and insect resistance. Once in the environment,
aldrin breaks down to dieldrin, another insecticide with a similar structure (U.S. EPA 2002a). It is not known
how much aldrin and dieldrin are presently stored in the U.S. (ATSDR 2002).
Use: Both aldrin and dieldrin were used worldwide as broad-spectrum insecticides to protect crops such as
cotton, corn, and citrus products, and for the extermination of termites. In Canada, aldrin and dieldrin were used
as pesticides until 1990 when permitted uses were cancelled (Health Canada, 1998). Aldrin was also used in the
U.S. as a soil insecticide to control root worms, beetles, and other crop pests, and as a treatment for timber,
plastic and rubber coverings to control termites and other pests. Aldrin use in the U.S. peaked in 1966 at 19
million pounds but had dropped to 10.5 million pounds by 1970. Because aldrin is not currently produced or
imported into the U.S., its use is believed to be minimal and any past releases have likely been converted to
dieldrin (U.S. EPA 2002a).
In Mexico, Aldrin and Dieldrin are employed principally to combat termites and to control insects particularly
those associated with public health. The use of these compounds has been greatly reduced and continues to
decrease. According to statistics from the Economic Secretariat, Mexico has imported (mostly from the U.S.
over 70%) 7,649 tonnes of Aldrin and it has exported 3,186 tonnes of this product to Costa Rica, Colombia and
the United States.
Emissions: U.S. industrial emissions of aldrin were inventoried by the TRI for the 2000 reporting year while
industrial emissions of dieldrin are not inventoried by the TRI (U.S. EPA 2002a).
U.S. industrial emissions of aldrin for 2000 are as follows (U.S. EPA 2002a):
Total on-site releases were 2,342.79 pounds (of these, releases to air were 0.79 pounds and to land were 2,342
pounds) and total off-site releases (transfers to disposal) were 2.58 pounds. Total on- and off-site releases were
2,345.37 pounds.
Aldrin and Dieldrin are not manufactured or used in Canada, and as such, Canadian industrial emissions of
aldrin and dieldrin are not inventoried by the NPRI (Environment Canada 2002b).
2.2.1.2 Endrin
Source: Endrin is a stereoisomer of dieldrin produced by the reaction of vinyl chloride and
hexachlorocyclopentadiene to yield a product which is then dehydrochlorinated and condensed with
27
cyclopentadiene to produce isodrin. This intermediate is then epoxidized with peracetic or perbenzoic acid to
yield endrin. An alternative production method involves condensation of hexachlorocyclopentadiene with
acetylene to yield the intermediate for condensation with cyclopentadiene. Endrin is no longer manufactured in
the United States. There was one U.S. company producing endrin until the final voluntary cancellation of
registration with the U.S. Office of Pesticide Programs in 1991. It is estimated that 2.345 million kg (5.1-9.9
million pounds) of endrin were sold in the United States in 1962, while less than 450,000 kg (990,000 pounds)
were produced in 1971 (ATSDR 2002).
More recent estimates of U.S domestic production of endrin could not be found.
Use: Endrin was first used as an insecticide, rodenticide and avicide beginning in 1951 to control cutworms,
voles, grasshoppers, borers, and other pests on cotton, sugarcane, tobacco, apple orchards, and grain. It was also
used as an insecticide agent on bird perches. Unlike aldrin/dieldrin, with which it has many chemical
similarities, endrin apparently was never used extensively for termite-proofing or other applications in urban
areas. Endrin's toxicity to nontarget populations of raptors and migratory birds was a major reason for its
cancellation as a pesticide agent. Except for use as a toxicant on bird perches, which was cancelled in 1991, all
other uses of endrin in the United States were voluntarily cancelled by the manufacturer in 1986. It has been
estimated that 6,250 kg (13,780 pounds) of endrin were used annually in the United States prior to 1983. Since
endrin may still be used as a pesticide agent in foreign countries, residues on imported food items are still of
some concern. Both the U.S. EPA and FDA revoked all food tolerances for endrin in 1993 (ATSDR 2002).
In Canada, most uses of endrin were phased out in the 1970s. The persistent nature of this insecticide prompted
periodic re-evaluations of its registration. By the mid-1970s, use of endrin was restricted to the control of
cutworms on cereals and minor pests on potatoes. In 1989, the registrant indicated that there would be no
further manufacture of the pesticide. The registration of endrin ended in 1990.
Emissions: U.S. industrial emissions of endrin are not inventoried by the TRI (U.S. EPA 2002a). Endrin is not
manufactured or used in Canada, and as such, Canadian industrial emissions of endrin are not inventoried by the
NPRI (Environment Canada 2002b).
2.2.1.3 Chlordane
Source: Chlordane was first marketed in 1948 in a variety of formulations (U.S. EPA 2002a). U.S. EPA
estimated that 3.5 - 4.0 million pounds of chlordane were distributed in 1986. There was only one U.S. domestic
manufacturer of chlordane at the time (ATSDR 2002). Concern over the health effects and particularly the
carcinogenicity of chlordane, lead to an eventual ban on all U.S. domestic uses of chlordane in 1988 (U.S. EPA
2002a). In 1997, the world's last producer of chlordane, the U.S.-based Velsicol Chemical Corporation,
announced that it would permanently cease production (UNEP 2000).
Chlordane was first registered in Canada in 1949 for the control of insect pests in crops and forests, as well as
for domestic and industrial applications. It was never manufactured in Canada (CEC 1998).
Use: In the United States, chlordane was once widely used as an insecticide on corn, citrus, and home gardens,
and as a fumigant in termite and carpenter ant control. In 1978, a cancellation notice was issued that banned all
uses of chlordane except for root dipping of non-food plants and underground treatment against termites (U.S.
EPA 2002a). As of April 14, 1988, all commercial use of chlordane in the U.S. was cancelled (ATSDR 2002).
The use pattern for chlordane in the mid 1970s was as follows: 35% used by pest control operators, mostly on
termites; 28% on agricultural crops, including corn and citrus; 30% for home lawn and garden use; and 7% on
turf and ornamentals (ATSDR 2002).
In Canada, most uses of chlordane were phased-out by the mid-1970s in response to environmental and safety
concerns. In December 1985, with the exception of its use to control subterranean termites by licensed pesticide
applicators, applications for the use of chlordane were suspended. Even its use against termites was voluntarily
discontinued by the registrant in 1990, with the understanding that the existing stock would be sold, used or
disposed-of by the end of 1995. After this date, any sale or use of chlordane in Canada represents a violation of
the Canadian Pest Control Products Act (CEC 1998).
In 2001, Mexico imported 588.47 tonnes chlordane, 59% of this was from the US (Secretaría de Economía).
28
Emissions: U.S. industrial emissions of chlordane inventoried by the TRI for the 2000 reporting year are as
follows (U.S. EPA 2002a):
Total on-site releases were 8,961.44 pounds (of these, releases to air were 13.70 pounds and on-site releases to
land were 8,947.74 pounds) and total off-site releases (transfers to disposal) were 828.59 pounds. Total on- and
off-site releases were 9,790.03 pounds.
Chlordane is not manufactured or used in Canada, and as such, Canadian industrial emissions of chlordane are
not inventoried by the NPRI (Environment Canada 2002b).
2.2.1.4 Heptachlor
Source: Heptachlor is an organochlorine insecticide, which was first isolated from technical chlordane in 1946.
Technical heptachlor is a mixture of pure heptachlor and many related chemicals. Heptachlor does not occur
naturally in the environment. It is a white powder that smells like mothballs (U.S. EPA 2002a).
Heptachlor is produced by the chlorination of chlordane. Technical heptachlor contains 20 percent chlordane
(U.S. EPA 2002a).
Heptachlor was first registered for use in the United States as an insecticide in 1952 and commercial production
began the following year. The sale of heptachlor was voluntarily cancelled in 1987 by its sole U.S.
manufacturer. The sale, distribution, and shipment of existing stocks of all cancelled chlordane and heptachlor
products were prohibited in the United States as of April 1988 (ATSDR 2002).
Use: In the United States, heptachlor was used extensively from 1953 to 1974 as a soil and seed treatment to
protect corn, small grains, and sorghum from pests. It was used to control ants, cutworms, maggots, termites,
thrips, weevils, and wireworms in both cultivated and uncultivated soils. Heptachlor was also used
nonagriculturally during this time period to control termites and household insects (ATSDR 2002).
Nearly all registered uses of heptachlor were cancelled in 1974 by the U.S. EPA because of its potential cancer
risk and its persistence and bioaccumulation throughout the food chain (ATSDR 2002). All uses were cancelled
in 2000 (U.S. EPA 2002a).
Heptachlor was widely used in Canada to control insect pests in crops, and for domestic applications. In
response to environmental concerns, most Canadian uses of heptachlor were phased out in the 1970s. The
persistent nature of this insecticide prompted periodic re-evaluations of its registration. With the exception of a
use on narcissus bulbs, all uses of heptachlor were suspended effective December 31 1976. The last use of
heptachlor on narcissus was voluntarily discontinued by the registrant as of December 31 1985. The registration
of heptachlor ended in 1985.
The Economic Secretariat reported that Mexico imported 816.20 tonnes of heptachlor in 2001 from Japan and
the US. During this period Mexico exported 261.77 tonnes.
Emissions: U.S. industrial emissions of heptachlor inventoried by the TRI for the 2000 reporting year are as
follows (U.S. EPA 2002a):
Total on-site releases were 2,379.16 pounds (of these, releases to air were 6.60 pounds and releases to land were
2,372.56 pounds) and total off-site releases (transfers to disposal) were 221.87 pounds. Total on- and off-site
releases were 2,601.03 pounds.
Heptachlor is not manufactured or used in Canada, and as such, Canadian industrial emissions of heptachlor are
not inventoried by the NPRI (Environment Canada 2002b).
2.2.1.5 Dichlorodiphenyltrichloroethane (DDT)
Source: The cumulative world production of DDT has been estimated at 2 million tons (ATSDR 2002). There
are still major producers of DDT in India and some Central and South American countries, where DDT is used
in malaria control. The World Health Organization (WHO) has concluded that DDT should not be used if an
alternative insecticide is available (Health Canada 1998).
Commercial production of DDT for agricultural use and vector control began in 1945, its use peaking in 1963.
In Canada, the use of DDT was restricted in 1974 and stopped in 1989 (Health Canada 1998). As of January 1,
29
1973, all uses of DDT in the United States were cancelled except emergency public health uses and a few other
uses permitted on a case-by-case basis (ATSDR 2002).
In México, DDT production by the state enterprise Fertilizantes Mexicanos (Fertimex) started 1968 with peak
production reaching eighty thousand tons (80,000 tonnes.) per year. In 1991, when Fertimex was privatized, the
production and exportation by the new enterprise Tekchem continued until recently when production ceased.
Use: DDT was first registered in 1946 and used in Canada to control insect pests in crops as well as in domestic
and industrial applications, DDT was never manufactured in Canada. DDT is a chemical of anthropogenic origin
first used for the control of insects capable of transmitting malaria, typhus, and other insect vectors of diseases
during World War II (Health Canada 1998). In response to environmental and safety concerns, most uses of
DDT were phased out by the mid-1970s. Registration of all remaining uses of DDT was discontinued in 1985
with the understanding that existing stocks would be sold, used or disposed of by December 31, 1990. After this
date, any sale or use of DDT in Canada represents a violation of the Canadian Pest Control Products Act (CEC
1998).
In 1973, all uses of DDT in the United States were cancelled with the exception of public health use for control
of vector-borne diseases, USDA or military use for health quarantine, and use in prescription drugs for
controlling body lice. In 1988, all uses were cancelled and DDT is currently not manufactured in the United
States (CEC 1998).
DDT usage in Mexico has been decreasing for several decades. These actions, initiated by the Health
Secretariat of Mexico, were strengthened when DDT was included in one of the North American Regional
Action Plans (NARAP) of the CEC. The target was to reduce DDT usage by 80% by 2002. The NARAP is
nearly complete and the target was exceeded in that DDT use was completely eliminated by 2000. The
decreased use of DDT in Mexico is illustrated in Figure 4:
5 0 0 0
4 5 0 0
4 0 0 0
3 5 0 0
3 0 0 0
S
E
N
2 5 0 0
TON
2 0 0 0
1 5 0 0
1 0 0 0
5 0 0
0
*
63
73
5
83
5
5
1959
1961
19
1965
1967
1969
1971
19
197
1977
1979
1981
19
198
1987
1989
1991
1993
199
1997 1999
Y E A R S
Figure 4: Mexico DDT Usage (Tonnes)
Emissions: U.S. industrial emissions of DDT are not inventoried by the TRI (U.S. EPA 2002a). DDT is not
manufactured or used in Canada, and as such, Canadian industrial emissions of DDT are not inventoried by
NPRI (Environment Canada 2002b).
2.2.1.6 Toxaphene
Source: Toxaphene is not a naturally occurring compound. It is a contact insecticide consisting of a complex
mixture of over 670 chemicals. It was widely used on cotton crops, cereals, grains, fruits, nuts, oil seeds and
vegetables; as a piscicide in fish eradication programs; and on livestock and poultry. Toxaphene use began in
1949. Since 1984, toxaphene has been banned from use in Canada; all maximum residue limits in foods were
revoked (Health Canada 1998).
30
In 1982, the U.S. EPA cancelled the registrations of toxaphene for most uses as a pesticide or pesticide
ingredient, except for certain uses under specific terms and conditions. Since all registered uses of toxaphene on
food commodities were cancelled by 1990, and the sale and use of existing stocks in the United States were
prohibited after March 1, 1990, production of toxaphene for all domestic uses in the United States has ceased.
However, U.S. chemical manufacturers can legally produce pesticides for export that are currently banned or not
registered for use in the United States (ATSDR 2002).
Use: Toxaphene is an insecticide that was primarily used in the southern U.S. to control pests on cotton,
vegetables, livestock and poultry, soybeans, and alfalfa, wheat, and sorghum. Its relatively low toxicity to bees
and its long-persisting insecticidal effect made it particularly useful in the treatment of flowering plants
(ATSDR 2002). All registered uses of toxaphene in the U.S. were cancelled in 1990. It is still commonly used as
an insecticide on bananas and pineapples in Puerto Rico and the Virgin Islands (U.S. EPA 2002a).
Emissions: U.S. industrial emissions of toxaphene inventoried by the TRI for the 2000 reporting year are as
follows (U.S. EPA 2002a):
Total on-site releases were 5,950.83 pounds (of these, releases to air were 20.98 pounds, releases to water were
1.62 pounds, underground injection were 0.21 pounds, and on-site releases to land were 5,928.02 pounds) and
total off-site transfers to disposal were 176.14 pounds. Total on- and off-site releases were 6,126.97 pounds.
Toxaphene is not manufactured or used in Canada, and as such, Canadian industrial emissions of toxaphene are
not inventoried by the NPRI (Environment Canada 2002b).
2.2.1.7 Mirex
Source: Mirex does not occur naturally in the environment. It was first manufactured in 1946, primarily for use
as an insecticide to control fire ants. Although it was originally synthesized in 1946, mirex was not
commercially introduced in the United States until 1959 (ATSDR 2002).
Use: Mirex was used in pesticide formulations and as an industrial fire retardant. Because it is non-flammable,
mirex was marketed primarily as a flame retardant additive in the United States from 1959 to 1972 for use in
various coatings, plastics, rubber, textiles, paint, paper, and electrical goods. Several formulations of mirex have
been prepared in the past for various pesticide uses. Mirex was most commonly used in the U.S. in the 1960s as
an insecticide to control the imported fire ants. From 1962 to 1976, in the U.S., approximately 132 million acres
(53.4 million hectares) in 9 states were treated with approximately 485,000 pounds (226,000 kg) of mirex at a
rate of 4.2 g/hectare (later reduced to 1.16 g/hectare). All U.S. registered products containing mirex were
effectively cancelled on December 1, 1977. However, selected ground application was allowed until June 30
1978, at which time the product was banned in the United States with the exception of continued use in Hawaii
on pineapples until stocks on hand were exhausted (ATSDR 2002).
Although use of mirex in Canadian agriculture was never permitted, it has been imported into Canada for other
uses. All uses of mirex have been banned in Canada since 1978 (Health Canada 1998).
Emissions: U.S. industrial emissions of mirex are not inventoried by the TRI (U.S. EPA 2002a) and Canadian
industrial emissions of mirex are not inventoried by the NPRI (Environment Canada 2002b).
2.2.1.8 Hexachlorobenzene (HCB)
Source: Hexachlorobenzene (HCB) is no longer manufactured as a commercial end product in the United
States, and evidence indicates that it has not been commercially produced since the late 1970s. However,
hexachlorobenzene may still be produced for on-site use and processing, as a by-product, or as an impurity
during the manufacturing of the pesticides ametryn, atrazine, cyanazine, dacthal, dienochlor, dipropetryn,
lindane, maleic hydrazide, mirex, pentachloronitrobenzene, picloram, prometon, prometryn, propazine,
simazine, and terbutryn (ATSDR 2002). The products which generally contain detectable levels of HCB are
dacthal, pentachloronitrobenzene, picloram and pentachlorophenol. In Canada, HCB has not been used as a
commercial chemical since 1972. Small quantities of HCB were imported into Canada during the 1980s (Health
Canada 1998). Currently, the principal sources of hexachlorobenzene to the Canadian environment are estimated
to be, application of HCB-contaminated pesticides, waste incineration (including municipal solid waste) and
long-range transport from other countries. Metallurgical processes also emit some HCB in North America
31
(Bailey 2001). The highest concentrations of HCB have been observed near point sources in the Great Lakes and
connecting channels. Hexachlorobenzene is a widely distributed substance in the Canadian environment. It has
been detected in air, water, sediment, soil and biota (CEPA 1993).
Use: HCB was once used as an agricultural fungicide, but health concerns about the toxicity of HCB led to the
cancellation of the registrations of all pesticides that contained HCB as an active ingredient (U.S.EPA 2002a).
There are no current commercial uses of hexachlorobenzene as an end-product in the United States (ATSDR
2002). Its primary use was as a fungicide on the seeds of onions, sorghum, wheat, and other grains until 1984,
when the last registered use of the compound as a pesticide was voluntarily cancelled. Historically,
hexachlorobenzene was also used in the production of pyrotechnic and ordinance materials for the military, the
production of synthetic rubber, as a porosity controller in the manufacture of electrodes, a chemical intermediate
in dye manufacturing, and a wood preservative (ATSDR 2002).
Emissions: U.S. industrial emissions of hexachlorobenzene inventoried by the TRI for the 2000 reporting year
are as follows (U.S. EPA 2002a):
Total on-site releases were 24,506.26 pounds (of these, releases to air were 1,426.24 pounds, releases to water
were 331.44 pounds, underground injection were 48.39 pounds, and releases to hazardous waste landfills were
22,700.20 pounds) and total off-site releases (transfers to disposal) were 13,021.04 pounds. Total on- and off-
site releases were 37,527.30 pounds of which approximately 1,426 plus 331 pounds could be expected to be
mobile in the environment.
Canadian industrial hexachlorobenzene emissions were inventoried by the NPRI for the 2000 reporting year and
are available through the Query Site (NPRI Query 2002)). Total on-site releases were 37.80 kg, transfers for
disposal 10.45 kg, transfer for recycling 0.25 kg.
2.2.2 Industrial Compounds - Polychlorinated Biphenyls (PCBs)
Source: Polychlorinated biphenyls, otherwise known as PCBs, were first created in 1881, and commercial
manufacture began in 1929. They do not occur naturally. PCBs are a family of 209 compounds (referred to as
congeners) with similar structures (Health Canada 1998). In the U.S., more than 1.25 billion pounds of PCBs
were produced from 1930 to 1975. Domestic U.S. production of PCBs was banned in 1976 under the Toxic
Substances Control Act (TSCA) (U.S. EPA 2002a). Marketed worldwide under trade names such as Aroclor,
Askarel, and Therminol, the annual U.S. production peaked in 1970 with a total production volume of 85
million pounds (39 million kg) of Aroclor (ATSDR 2002).
PCBs are released to the environment from improper disposal practices and accidental releases (Health Canada
1998).
In México most PCB's were imported from Monsanto Chemical Corporation until they were closed in the
1970´s. There were also minor quantities imported from Europe and Japan. In México PCBs still exist in
transformers, electric capacitors and ballast, some of which have been in operation since before 1980.
Use: PCBs have been used in capacitors and transformers, hydraulic fluids, adhesives, plasticizers, heat transfer
fluids, wax extenders, lubricants, cutting oils and flame retardants (Health Canada 1998; U.S. EPA 2002a;
ATSDR 2002). Concerns over the environmental effects of PCBs led to a North American ban in 1977 on their
manufacture, importation and most non-electrical uses and also to restrictions on their use in existing electrical
and mechanical equipment. Despite these actions, PCBs are still in use today from applications previous to their
restriction (Health Canada 1998).
In 1976, U.S. EPA estimated that 250,000 U.S. tons had already entered the environment and that 375,000 U.S.
tons remained in electrical equipment. By 1988, U.S. EPA estimated that 141,000 U.S. tons of pure PCBs still
remained in use. In addition, U.S. EPA estimates that 26 million cubic meters of soils are contaminated with
PCBs (CEC 1998).
At the end of 2001, the Canadian federal government's nationwide PCB inventory included approximately
30,822 tons (27,961 tonnes) of PCBs and PCB material in-use (excluding fluorescent lamps), and 140,050
116,922 tons (106,070 tonnes) of PCBs, PCB material, and contaminated soil in storage. There is, presumably, a
32
large but relatively unknown quantity of fluorescent lamp ballasts in use, which is not in the national inventory.
(Personal Communication: Guy Gagne, Environment Canada).
In México, PCB use has been primarily in transformers, electric capacitors and fluorescent lights ballast. Minor
uses were hydraulic fluid, the automotive industry, printing inks, marine painters and carbon copies paper.
Currently, there is not enough information to establish precise quantities and locations of these compounds.
Nonetheless, in 1995, the National Institute of Ecology (INE) generated the first preliminary inventory, which
indicated that about 12, 400 tons of these compounds existed in The Mexican Republic. However, it is notable
that between 1995 and 2001, 8,361 tonnes of PCB's were exported for destruction to Germany, Spain, U.E.A.,
Finland, Holland and England. An improved inventory was published recently by INE, revealing that about
9,374 tonnes of PCBs remain in Mexico.
Emissions: U.S. industrial emissions of PCBs inventoried by the TRI for the 2000 reporting year are as follows
(U.S. EPA 2002a):
Total on-site releases were 1,434,770.77 pounds (of these, releases to air were 5,854.15 pounds, releases to
water were 28.82 pounds, underground injection were 0.60 pounds, and on-site releases to land were
1,428,887.20 pounds) and total off-site releases (transfers to disposal) were 26,146.07 pounds. Total on- and off-
site releases were 1,460,916.85 pounds.
Total U.S. polychlorinated biphenyls (PCBs) in industrial production-related waste were projected to decrease
from 2000 to 2002 by 0.7 percent (U.S. EPA 2002a).
Canadian industrial emissions of PCBs are not inventoried by the NPRI (Environment Canada 2002b).
2.2.3 Unintended By-products - Polychlorinated dibenzo-p-dioxins (PCDDs) and Polychlorinated
dibenzofurans (PCDFs)
Source: Dioxins and furans [chlorinated dibenzodioxins (CDDs), chlorinated dibenzofurans (CDFs)] are two
chemical families closely related by their structural formulae. There are 75 dioxins and 135 furans. The most
toxic, and the most studied of these chemicals is 2,3,7,8-tetrachlorodibenzodioxin (2,3,7,8-TCDD or TCDD)
(Health Canada 1998; U.S. EPA 2002a).
Although produced through natural sources (volcanoes, forest fires), the majority of dioxins are anthropogenic.
Dioxins and Furans are unintentional by-products of certain industrial, non-industrial and natural processes,
usually involving combustion. Energy generation sources of CDD/CDF include emissions from the combustion
of coal, oil and petroleum-based fuels, and wood in residential, industrial, and electric utility establishments.
Industrial combustion of these fuels occurs in all of the manufacturing sectors. Other relatively minor, high-
temperature sources include metal smelting, Portland cement production, pulp mills using the kraft process,
asphalt mixing plants, catalyst regeneration at petroleum refineries, carbon reactivation furnaces and cigarette
smoking. In addition, minimally controlled or uncontrolled combustion sources may emit CDD/CDFs, including
fires and spills involving PCBs (U.S. EPA 2002a).
Pentachlorophenol (include tetrachlorophenol) is still used in Canada to preserve and protect wood. Although in
1981 this compound was one of the largest potential sources of octachlordibenzodioxin to the environment, the
dioxin content and the amounts used have since been reduced. Consequently, today its potential as a source is
only one tenth of that noted earlier by the National Research Council of Canada (NRCC), and this is likely to
decrease further as pentachlorophenol is replaced by alternative wood protection chemicals or processes in the
near future (CEPA 1990).
Overall, levels of dioxins and furans in the environment have declined significantly over the past 25 years.
Better combustion practices will have the biggest effect on further reducing environmental levels of dioxins and
furans from known sources (CCC 2002).
Use: Furans are a trace contaminant of PCBs. Although the use and storage of these compounds is now strictly
controlled, fires and spills involving PCBs remain a source of furan contamination (Health Canada 1998).
33
In Canada, the sale and use of pesticides containing CDD is not permitted. The Government of Canada, in
conjunction with the provinces, has established codes of practice to reduce contamination by the wood
preservation and protection industries (Health Canada 1998).
Emissions: U.S. industrial emissions of CDDs and CDFs (collectively referred to as dioxin and dioxin-like
compounds by the U.S. TRI) inventoried by the TRI for the 2000 reporting year are as follows (U.S. EPA
2002a):
Total on-site releases were 101.24 pounds (of these, releases to air were 11.51 pounds, releases to water were
4.58 pounds, underground injection were 0.90 pounds, and releases to land were 84.27 pounds) and total off-site
transfers to disposal were 118.85 pounds. Total on- and off-site releases were 220.09 pounds.
The TRI inventory provides a partial evaluation of dioxin emissions in the U.S. An alternate comprehensive and
scientifically based approach to estimating actual emissions of dioxins has been undertaken by the U.S. EPA
through its Database of Sources of Environmental Releases of Dioxin-Like Compounds in the United States
(www.cfpub.epa.gov/ncea/cfm/dioxindb.cfm). This approach shows that quantified emissions (those which
could be estimated with some confidence) fell from more than 13,000 g TEQ per year in 1987 to approximately
3,300 g TEQ per year in 1995. U.S. EPA projects a further decline to approximately 1,500 g TEQ in 2005.
(U.S. EPA, 2000c). The majority of the reductions are due to regulations controlling emissions from municipal
waste incinerators. Reductions in these and other industrial sources have increased the relative importance to
total emissions of the dioxins emitted from uncontrolled sources including open burning of rubbish, landfill fires
and forest fires.
The Canadian Government's priority substances assessment report for polychlorinated dibenzodioxins and
polychlorinated dibenzofurans concluded that polychlorinated dibenzodioxins and polychlorinated
dibenzofurans may enter the environment in quantities which have immediate and long-term harmful effects on
the environment, and which constitute a danger in Canada to human health. These substances are therefore
considered "toxic" as defined under Sections 11(a) and 11(c) of the Canadian Environmental Protection Act
(Environment Canada 2002f).
In February 2001, Environment Canada released an Up-dated Edition of Inventory of dioxin/furan releases (see
http://www.ec.gc.ca/dioxin/english/inventory.cfm ). Releases to air are estimated at 164 ITEQ in g/y, releases to
water at 3 ITEQ in g/y, releases to soil at 19 ITEQ in g/y and quantity in solid waste at 1097 ITEQ in g/y.
Canadian industrial dioxin/furan emissions were inventoried by the NPRI for the 2000 reporting year and are
available through the Query Site (NPRI Query 2002).
Emissions from open, uncontrolled combustion of organic matter are not considered. These growing sources
include barrel burning, agriculture (e.g. stubble burning) and wood burning for home heating and cooking.
Dioxins, furans, dioxin-like coplanar PCB's and hexachlorobenzene are products of concern.
A preliminary inventory of dioxin/furan emissions was prepared for Mexico in 2001 (Autonomous Metropolitan
University 2001). It identifies the principle sources and estimates of emissions and trends of both natural and
anthropogenic sources including waste combustion, cement, chemical and metallurgical industries as well as
other key sectors.
2.2.4 Regionally Specific PTSs
These chemicals are included in the UNEP list of 26. In North America, the following are receiving attention:
HCH, Chlordane, Endosulfan, PCP and PAHs.
2.2.4.1 Hexachlorocyclohexanes (HCH)
Source: Hexachlorocyclohexane isomers (HCH) do not occur as a natural substance. The manufacture of
technical-grade HCH involves the photochlorination of benzene, which yields an isomeric mixture consisting of
-HCH, -HCH, -HCH, -HCH, -HCH, and inert S-isomers. This reaction can be started by free-radical
initiators such as visual or ultraviolet light, X-rays, or -rays. Treatment with methanol or acetic acid, followed
by fractional crystallization, concentrates -HCH to the 99.9% required in the technical-grade of -HCH; nitric
acid is used to remove odour. None of the isomers or technical-grade HCH is currently produced in the United
States. The production of -HCH (lindane) exceeded 2.27×106 g in 1976; commercial -HCH production in the
34
United States reportedly ended in that year. However, the Directory of Chemical Producers for 1987 and 1988
lists one producer of -HCH; subsequent years (19891991) give no listings of -HCH producers (ATSDR
2002).
Use: Lindane usage in Canada in 1968 was reported to be 16 t (tonnes, t, are metric tonnes), decreasing to 3 t in
1969 and 1970 and 2.8 t in 1971 (Voldner and Smith, 1986), however, usage may have been underestimated.
During the 1990s lindane imports of 100-150 t/y were reported (Barrie et al. 1997). Lindane became one of top
ten insecticides used in Canada during the 1990s (Environment Canada 1992). However, pesticide information
from Canadian companies is proprietary. Thus, surrogate cropland information was used to estimate lindane
usage for Canada, as outlined below. The majority of lindane applications have been on canola seed in the
prairie region of Canada, and on corn seed in eastern Canada (CACAR 2002).
In Canada, following a recent special review of lindane and the status of lindane registrations, all sale of lindane
products for use on canola/rapeseed, and all use of lindane-treated canola seed voluntarily ceased July 1, 2001,
and carry-over of lindane-treated seed were planted for the spring 2002 planting season only. This was the final
use season for lindane-treated canola seed, and represented approximately 90% of lindane use in Canada. Of the
remaining uses, all use of lindane seed-treatment products and planting of lindane-treated seed of wheat, barley,
oats, rye, flax, corn, beans, soybeans and peas are prohibited after December 31, 2004. All use of lindane seed-
treatment products and planting of lindane-treated seed for mustard, cabbage, broccoli, Brussels sprouts,
cauliflower and rutabaga will be prohibited after October 1, 2002.
As in Canada, pesticide information from the U.S. companies is proprietary. However, information on
agricultural pesticide use is available from several national pesticide use surveys and reports, along with a
variety of research reports on specific crops or states. For example, the U.S. Department of Agriculture's
(USDA) National Agricultural and Statistics Service (NASS) provides annual state summaries of pesticide use
for major field crops and the United States Census of Agriculture conducts a survey every 5 years (CACAR
2002).
Of particular value is the 1995 database of the National Center for Food and Agricultural Policy (NCFAP), a
non-profit, nongovernmental organization based in Washington, D.C. Its National Pesticide Use Database
contains over 15,000 individual records that quantify the use of specific active ingredients by crop and state
(NCFAP 2000). The database contains use estimates for 200 active ingredients, including lindane, used on 87
crops in the 48 contiguous states for a typical crop year in the 1991-1993 time period (CACAR 2002).
Although the NCFAP database offers a unique source of pesticide use data for the United States, the data of
lindane are for crop spray only and seed dressing is not included. In estimates below, the use of lindane for seed
dressing is not addressed due to lack of information. (CACAR 2002)
Technical HCH use in the U.S. began the late 1940s and lindane followed afterward. In 1978, technical HCH
was banned while lindane usage continued (Barrie et al., 1992). As noted above, U.S. information is limited
regarding use patterns of lindane. The import of lindane to the United States was 1.6 kt in 1974, 2.9 kt in 1975,
13.1 kt in 1976, and 152 kt in 1977 (Voldner and Smith, 1986), an increase of almost a factor of 100 over four
years. The great increase in lindane import in 1977 could be due to the ban of technical HCH in following year
(CACAR 2002).
Emissions: U.S. industrial emissions of HCH are not inventoried by the TRI (U.S. EPA 2002a) and Canadian
industrial emissions of HCH are not inventoried by the NPRI (Environment Canada 2002b).
2.2.4.2 Chlorinated Paraffins (CPs)
Source: Chlorinated Paraffins (CPs) are a family of complex mixtures (WHO 1996). While more than 200
commercial products (CPIA 2001) have been reported, CPs are typically grouped into categories based on
average degree of chlorination and their carbon chain-length (short- C10-13, medium- C14-17 and long- C18-
30). There are three commercial manufacturers of chlorinated paraffins in North America; two manufacturing
facilities in the US and one in Canada (CPIA 2001 update).
The Mexican Economic Secretariat has reported that in 2000 and 2001, Mexico imported 46,942 and 40,481
tonnes of CPs, respectively. In 2002 (until August) Mexico imported approximately 23,000 tonnes of CPs.
35
More than 80% of these compounds come from the US. Mexico exported 358.31 tonnes (January-August 2002)
of these substances to Chile, Colombia, USA, Peru and Venezuela.
Use: In the Region, use applications for chlorinated paraffins range from extreme pressure additives in
lubricants, to secondary plasticizers in paints and plastics, to flame retardants in various plastics and textiles
(Environment Canada. 2002a, CPIA 2001). The overwhelming use of CPs in North America is as additives in
cutting oils and high pressure lubricating oils where the requirements for chemical stability are high. At high
temperatures, these oils react to form low melting inorganic lubricant films on metal surfaces. This film prevents
unwanted welding of metal parts (CPIA 2001).
In the plastics industry, chlorinated paraffins are often used as secondary plasticizers in polyvinyl chloride
(PVC), where CPs enhance the plasticizing effects and are thus also known as "extenders." Secondary
plasticizers are used in combination with primary plasticizers, such as phthalates and phosphate esters.
Chlorinated paraffins can be used as well in other plastics including flexible vinyl in the housing and automobile
industries, acrylonitrile-butadiene-styrene resins (ABS), unsaturated polyester resins, polyethylene,
polypropylene and urethane foam (CPIA 2001).
To a lesser degree, CPs are also commonly found in rubbers, paints, adhesives, caulks and sealants, and function
as either plasticizers or flame retardants (CPIA 2001).
In the Region, demand in 2000 for all CPs was estimated to be is approximately 48,000 tons/yr. The use of
short-chain CPs was estimated to be 5,500 tons. Global consumption of all chlorinated paraffins was
approximately 300,000 tons/yr in 1985. (WHO, 1996)
Emissions: On November 30, 1994, EPA expanded the list of chemicals subject to the Toxics Release Inventory
(TRI) reporting requirements. Included on the expanded list was a category of "polychlorinated alkanes (C10-
13)", the bulk of which are short-chain chlorinated paraffins (CPIA 2001 update, U.S. EPA 2002a). This
category is broader than short-chain chlorinated paraffins as reporting is required for all short-chain (10 to 13
carbons) whether or not the starting material is n-alkane (paraffin).
According to the TRI results for calendar year 2000, approximately 6,000 pounds were released to air and 5,700
pounds to water.
The Canadian Government's priority substances assessment report for chlorinated paraffins concluded that short
chain chlorinated paraffins are considered to be "toxic" as defined under Paragraph 11(c) of the Canadian
Environmental Protection Act. Available data are considered inadequate to evaluate whether medium and long
chain chlorinated paraffins are considered to be "toxic" as defined under Paragraphs 11(a) or (c) of the Canadian
Environmental Protection Act (Environment Canada 2002g).
On April 24, 1999, Environment Canada added short-chain chlorinated paraffins (SCCPs) to the list of
chemicals subject to reporting pursuant to the Canadian National Pollutant Release Inventory. Very few reports
have been filed in response to the NPRI requirements likely because there is minimal use of SCCP in Canada
(Environment Canada 2002b).
2.2.4.3 Chlordecone
Source: Chlordecone is an organochlorine that is used as an insecticide (PANNA 2002). Chlordecone was used
as an insecticide on tobacco, ornamental shrubs, bananas, and citrus trees, and in ant and roach traps
(Government of Canada 2002). Chlordecone is not known to occur in the environment as a natural product. The
synthesis of chlordecone was first reported in 1952. Chlordecone was introduced commercially in the United
States in 1958 under the trade names Kepone® and GC-1189. The major form of chlordecone, which was used
as a pesticide on food products, was a wettable powder (50% chlordecone). Chlordecone is no longer produced
commercially in the United States. Between 1951 and 1975, approximately 3.6 million pounds (1.6 million kg)
of chlordecone were produced in the United States (ATSDR 2002).
Use: Until August 1, 1976, chlordecone was registered in the United States for use on banana root borer (in the
U.S. territory of Puerto Rico); this was its only registered food use. Additional registered formulations included
non-food use on non-fruit bearing citrus trees to control rust mites; on tobacco to control tobacco and potato
wireworms; and for control of the grass mole cricket, and various slugs, snails, and fire ants in buildings, lawns,
36
and on ornamental shrubs. The highest reported concentration of chlordecone in a commercial product was 50%,
which was used to control the grass mole cricket in Florida. Chlordecone has also been used in household
products such as ant and roach traps at concentrations of approximately 0.125%. The concentration used in ant
and roach bait was approximately 25%. All registered products containing chlordecone were effectively
cancelled as of May 1, 1978 (ATSDR 2002).
Emissions: U.S. industrial emissions of chlordecone are not inventoried by the TRI (U.S. EPA 2002a) and
Canadian industrial emissions of chlordecone are not inventoried by the NPRI (Environment Canada 2002b).
2.2.4.4. Endosulfan
Source: Endosulfan is sold as a mixture of two different forms of the same chemical (referred to as alpha- and
beta-endosulfan). It is a cream-to-brown-coloured solid that may appear crystalline or be in flakes. It has a
distinct odour similar to turpentine. Endosulfan does not burn (ATSDR 2002).
Endosulfan was first introduced into the United States in 1954 by Farbwerke Hoechst A.G. under the registered
trademark, "Thiodan®". Several formulations containing endosulfan are presently on the market, and pesticide
manufacturers make use of various inert ingredients (such as alcohol solvent emulsifiers; petroleum distillate
emulsifiers; suspension agents, water, clay, and wetting agents; and talc) to produce these formulations.
Formulated (processed) endosulfan exists in several forms (most of which are registered under the name
"Thiodan®"). The main forms are the following: wettable powder with 17.5, 35, or 50% active ingredient
(technical) with clay and wetting agents as inert ingredients; and emulsifiable concentrate with 17.5 or 35%
active agent mixed with petroleum distillates or alcohol plus emulsifiers (as inert ingredients). Epichlorohydrin
was reported to have been used in technical-grade endosulfan at one time as a stabilizer. However, it is unclear
when this practice was discontinued (ATSDR 2002).
Few details are available on endosulfan's production volume. In 1974, the annual production of endosulfan in
the United States was estimated at 3 million pounds. However, domestic production was near 5,000 pounds in
1977. Endosulfan has not been produced in the United States since 1982; therefore, worldwide production
volumes listed after 1982 do not include data for the United States (ATSDR 2002).
Although endosulfan is no longer produced in the United States, it is still used in chemical formulations. No
production volume data were available for the companies that produce these formulations. From the available
information, it is unclear whether these sites represent producers of technical-grade endosulfan or manufacturers
of endosulfan formulations. There is currently only one facility that processes endosulfan in the United States
(ATSDR 2002).
Endosulfan is used as an insecticide in Canada (Environment Canada 2001a). Imports of endosulfan into the
United States for 1982 were estimated at 182,000 kg. Technical endosulfan is no longer produced in the United
States; therefore, it is no longer exported. Data on export of formulated products containing endosulfan were not
located (ATSDR 2002).
Use: Endosulfan is a man-made insecticide. It is used for control of a number of insects on food crops such as
grains, tea, fruits, and vegetables and on non-food crops such as tobacco and cotton. It is also used as a wood
preservative (ATSDR 2002).
Endosulfan is registered in the United States as a contact and stomach insecticide for over 60 food and non-food
crops. It is applied to crops to control over 100 different insect pests. Endosulfan formulations are used in
commercial agriculture and home gardening. They are also used for wood preservation. In the United States,
endosulfan is mainly applied to tobacco and fruit crops (ATSDR 2002).
Emissions: U.S. industrial emissions of endosulfan are not inventoried by the TRI (U.S. EPA 2002a) and
Canadian industrial emissions of endosulfan are not inventoried by the NPRI (Environment Canada 2002b).
2.2.4.5 Pentachlorophenol (PCP)
Source: Pentachlorophenol is a man-made substance. There are two manufacturers of pentachlorophenol in
North America: Vulcan Chemicals, a business unit of Vulcan Materials Company, which operates a PCP
production facility in Wichita, Kansas and KMG-Bernuth, Inc., which operates its PCP production facility in
37
Matamoros, Mexico. At one time, it was one of the most widely used biocides in the United States (ATSDR
2002).
U.S. production volumes for 1983-1986 were: 45 million pounds in 1983; 42 million pounds in 1984; 38 million
pounds in 1985; and 32 million pounds in 1986. More recent production data are not available (ATSDR 2002),
however, the US Pentachlorophenol Task Force estimates that the two North American manufacturers produced
18.2 million pounds in 1996 (Personal communication).
Based on figures from the early 1990s, the most important source of chlorophenol release in Canada (over 70
percent) is from treated products. Of this amount, roughly two thirds comes from industrial sources (leachate
from treated products, leaked drilling fluids, and spillage from in-service wood treatment) (Health Canada
1998).
Use: In the USA, PCP is no longer available to the general public and its purchase and use are restricted to
certified applicators. It is now used as an industrial wood preservative for power line poles, cross arms, fence
posts, and the like (ATSDR 2002).
The chlorophenols most often used in Canada are pentachlorophenol (PCP) and tetrachlorophenol (T4CP).
These are used solely as wood preservatives (pressure-treating timber for telephone poles). The use of
chlorophenols in sapstain control, for in situ groundline treatment of existing utility poles, and in most specialty
applications (paints, stains, wood joinery products, industrial water treatment products, oil field biocides and
material preservatives) were terminated on December 31, 1990 (Health Canada 1998).
Emissions: U.S. industrial emissions of PCP inventoried by the TRI for the 2000 reporting year are as follows
(U.S. EPA 2002b):
Total on-site releases were 1,937 pounds (of these, releases to air were 456 pounds, releases to water were 1,206
pounds, underground injection were 250 pounds, and releases to land were 25 pounds) and total off-site releases
(transfers to disposal) were 1,962 pounds. Total on- and off-site releases were 3,899 pounds.
Canadian industrial emissions of PCP are not inventoried by the NPRI (Environment Canada 2002b).
2.2.4.6 Hexabromobiphenyl (HxBB)
Source: The commercial production of PBBs began in 1970. Approximately 13.3 million pounds of PBBs were
produced in the United States from 1970 to 1976. Hexabromobiphenyl constituted about 11.8 million pounds
and octa- and deca-bromobiphenyl about 1.5 million pounds of this total. Over 98% of the hexabromobiphenyl
was produced as FireMaster BP-6 and the residual as FireMaster FF-1. The sole producer of hexabromobiphenyl
in the United States stopped producing this PBB in 1975 (ATSDR 2002).
Use: Hexabromobiphenyl is a polybrominated biphenyl (PBB) and was used as flame retardant additives in
synthetic fibres and molded plastics (Government of Canada 2002). Prior to termination of production,
hexabromobiphenyl was used as a fire retardant mainly in thermoplastics for constructing business machine
housings, and industrial (e.g., motor housing) and electrical (e.g., radio and TV parts) products. Smaller amounts
were used as a fire retardant in coating and lacquers and in polyurethane foam for auto upholstery (ATSDR
2002).
Emissions: U.S. industrial emissions of hexabromobiphenyl are not inventoried by the TRI (U.S. EPA 2002a)
and Canadian industrial emissions of hexabromobiphenyl are not inventoried by the NPRI (Environment Canada
2002b).
2.2.4.7 Polybrominated diphenyl ethers (PBDEs)
Source: There are many types of brominated flame retardants (BFRs); more than 75, including polybrominated
diphenyl ethers), which vary in their environmental, toxicological and physical characteristics. The European
Union Waste Electrical and Electronic Equipment (WEEE) Directive has chosen to focus its efforts on the two
classes of BFRs that pose the highest cause for concern, that is, polybrominated biphenyls (PBB) and
polybrominated diphenyl ethers (PBDE). These compounds will produce polybrominated dibenzodioxins and
polybrominated dibenzofuran, as well as poly brominated chlorinated dibenzodioxins and polybrominated
38
chlorinated dibenzofurans if incinerated under less than ideal conditions. (Environment Canada 2000). Modern
destruction technologies are capable of minimizing or preventing dibenzodioxin and dibenzofuran formation. In
1992, 40 million kilograms of PBDE were produced. In 1999, 67 million kilograms were produced worldwide
with the Americas accounting for approximately 50% (BSEF, 2000). There are three technical mixtures: penta,
octa and deca. Each mixture contains several congeners with the majority of them being penta, octa or deca,
respectively. The production volumes, uses and toxicology of the three products differ. The deca mixture
accounts for approximately 80% of total PDBE production. There are two major manufacturers of PBDEs,
Great Lakes Chemical is in the US, and Dead Sea Bromine is in Israel. In addition PBDEs are produced in
China, and India. (U.S. EPA Workshop 2001). An additional US manufacturer has been identified as
Albermarke Chemical Corporation (Personal Communication, American Chemistry Council 2002).
Use: Since the 1960s, three commercial PBDE formulations are in production. The pentabrominated product is
used principally to flame retard polyurethane foams in furniture, carpet underlay and bedding. The penta
technical mixture, which is associated with the highest human and environmental risk, has been banned in the
EU starting July 1, 2003. (U.S. EPA Workshop 2001). World-wide, in 1999 (BSEF, 2000), it accounted for
nearly 13 % of total PBDE production (8 500 kg) and is produced mainly in the USA (8 290 kg). Commercial
octa is a mixture of hexa- (10-12%), hepta- (44-46%), octa- (33-35%) and nonabromodiphenyl (10-11%) ethers.
It is used to flame retard a wide variety of thermoplastics and is recommended for injection moulding
applications such as high impact polystyrene (HIPS). The deca product (a single congener) is used
predominantly for textiles and denser plastics such as housings for a variety of electrical products in particular
TVs and computers.
Emissions: DecaBDE (and tetrabromobisphenol A, a widely-used flame retardant in printed wiring boards) are
included in the TRI. Decabromodiphenyl oxide (which is another name for decaBDE) is included on the NPRI.
The other PBDEs are not included on the NPRI or the TRI.
2.2.4.8 Polycyclic Aromatic Hydrocarbons (PAHs)
Source: There are both natural and man-made sources of PAHs (Environment Canada 2002b). There are more
than 100 different PAHs and generally occur as complex mixtures (for example, as part of combustion products
such as soot), not as single compounds (ATSDR 2002). Acenaphthene, acenaphthylene, and anthracene are
produced commercially in the United States (ATSDR 2002). Incidental sources from human activity include the
incomplete combustion of fossil fuels, organic matter and garbage; the industrial production of many petroleum
products, such as creosote, asphalt; cigarette smoke; and vehicle exhaust. Natural sources of PAHs include
forest fires, volcanoes and fossil fuels. PAHs are formed from fossil fuels through pyrolysis, a process in which
material is chemically changed at a high temperature, while the oxygen supply is restricted (Health Canada
1998).
Wood burning in residential homes is the largest anthropogenic source of PAHs (Health Canada 1998). In
Canada, forest fires, which release approximately 2000 tonnes of PAHs per year, are the single most important
natural source of PAHs. (CEPA 1994).
Use: There are no known uses for the PAHs acenaphthylene, benz[a]anthracene, benzo[b]fluoranthene,
benzo[e]pyrene, benzoblfluoranthene, benzo[k]fluoranthene, benzo[g,h,i]perylene, benzo[a]pyrene, chrysene,
dibenz[a,h]anthracene, indeno[ 1,2,3-c,d]pyrene, or pyrene, except as research chemicals (ATSDR 2002).
Anthracene is used as an intermediate in dye production, in the manufacture of synthetic fibres, and as a diluent
for wood preservatives. It is also used in smoke screens, as scintillation counter crystals, in organic
semiconductor research and to synthesize the chemotherapeutic agent, Amsacrine. Acenaphthene is used as a
dye intermediate, in the manufacture of pharmaceuticals and plastics, and as an insecticide and fungicide
(ATSDR 2002).
Emissions: U.S. industrial emissions of PAHs were inventoried by the TRI for the 2000 reporting year. The
PAHs that the TRI inventoried include Benz(a)anthracene, Benzo(b)fluoranthene, Benzo(j)fluoranthene,
Benzo(j,k)fluorene, Benzo(k)fluoranthene, Benzo(rst)pentaphene, Benzo(a)phenanthrene, Benzo(a)pyrene,
Dibenz(a,h)acridine, Dibenz(a,j)acridine, Dibenzo(a,h)anthracene, 7H-Dibenzo(c,g)carbazole,
39
Dibenzo(a,e)fluoranthene, Dibenzo(a,e)pyrene, Dibenzo(a,h)pyrene, Dibenzo(a,l)pyrene, 7,12-
Dimethylbenz(a)anthracene, Indeno[1,2,3-cd]pyrene, 3-Methylcholanthrene, 5-Methylchrysene, 1-Nitropyrene
(U.S. EPA 2002a).
U.S. industrial releases of PAHs for 2000 are as follows (U.S. EPA 2002b):
Total on-site releases were 2,261,361.11 pounds (of these, releases to air were 1,916,436.42 pounds, releases to
water were 18,137.05 pounds, underground injection were 10,000 pounds, and releases to land were 316,787.63
pounds) and total off-site releases (transfers to disposal) were 3,141,614.53 pounds. Total on- and off-site
releases were 5,402,975.63 pounds.
Total U.S. polycyclic aromatic compounds in industrial production-related waste were projected to decline by
12.0 percent from 2000 to 2002 (U.S. EPA 2002a).
Canadian industrial PAH emissions were inventoried by the NPRI for the 2000 reporting year and are available
through the Query Site (NPRI Query 2002).
The Canadian Government's priority substances assessment report for polycyclic aromatic hydrocarbons
concluded that polycyclic aromatic hydrocarbons are entering the environment in a quantity or concentration or
under conditions that may have harmful effects on the environment. Polycyclic aromatic hydrocarbons are not
considered to constitute a danger to the environment on which human life depends. The PAHs benzo[a]pyrene,
benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, and indeno[1,2,3-cd]pyrene may constitute a
danger in Canada to human life or health (Environment Canada 2002e).
The PAHs that the Canadian federal government inventoried includes Benzo(a)anthracene,
Benzo(a)phenanthrene, Benzo(a)pyrene, Benzo(b)fluoranthene, Benzo(e)pyrene, Benzo(g,h,i)perylene,
Benzo(j)fluoranthene, Benzo(k)fluoranthene, Dibenz(a,j)acridine, Dibenzo(a,h)anthracene, Dibenzo(a,i)pyrene,
7H-Dibenzo(c,g)carbazole, Fluoranthene, Indeno(1,2,3-c,d)pyrene, Perylene, Phenanthrene, Pyrene
(Environment Canada 2002b).
There were two PAHs with industrial emissions inventoried by the NPRI prior to 2000 that are considered PAHs
anthracene and naphthalene. For 1999, Canadian total on-site releases of anthracene was 1.587 tonnes and if
naphthalene was 59.057 tonnes (Environment Canada 2001b).
2.2.4.9 Phthalates
Source: Phthalates are man-made ester salts of alcohols and 1,2-benzenedicarboxylic acid, commonly known as
ortho-phthalic acid. They are structurally characterized by the chemical radical --C6H4[(COO)2]2-, and produced
at moderate cost in petroleum distillation operations, by the direct action on phthalic anhydride, of alcohols
generally derived from petroleum olefins. Diethylhexyl phthalate (DEHP), diisononyl phthalate (DINP) and
diisodecyl phthalate (DIDP), are subgrouped as general-purpose plasticizers, and di-n-butyl phthalate (DnBP or
DBP), butylbenzyl phthalate (BBP), di-n-hexyl phthalate DnHP or DHP), and di-n-octyl phthalate (DnOP or
DOP) are classified as specialty plasticizers. These are all dialkyl phthalate esters, meaning that both acidic
terminals of the phthalic structure are linked to straight or branched aliphatic chains. Release of phthalates
directly to the atmosphere, primarily as a result of their manufacture and their industrial uses, is believed to be
the most important mode of entry to the environment. Small amounts are also released through the incomplete
combustion of plastic materials (Health Canada, 1998).
Use: Phthalates represent the major plasticizers in the production of soft polyvinyl chloride (PVC) and vinyl
chloride resins. Such flexible PVC is used in many common items like toys, vinyl upholstery, shower curtains,
adhesives, coatings, and as components of paper and paperboard. Phthalate-plasticized PVC is also used in
medical devices applications such as the production of flexible tubing for administering parenteral solutions and
for hemodialysis treatment. Furthermore, phthalates are found in cap liners, printing inks on the outside of
packages, in PVC tubing for conveying liquid foods, but are no longer used in PVC films to wrap meat nor in
polypropylene containers. Uses of dibutyl phthalate include adhesives, coatings (including lacquers), cosmetics
(perfume solvent and fixative), suspension agents for solids in aerosols, lubricants for aerosol valves,
antifoamers, and skin emollients. At present there are two DEHP manufacturing facilities in Canada and it has
40
also been imported from the United States. DnOP and dibutyl phthalate are not produced in Canada, but are
imported for use (Health Canada, 1998).
Emissions: U.S. industrial emissions of phthalates inventoried by the TRI for the 2000 reporting year are as
follows (U.S. EPA 2002b):
Total on and off-site releases for dibutyl phthalate is 285,213 pounds,
di(2-ethylhexyl)phthalate is 1,361,449 pounds, and dimethyl phthalate is 360,527 pounds.
Although Canadian industrial emissions of various phthalates were inventoried by the NPRI for the 2000
reporting year, at the time of the writing of this report, no formal report had been published by the NPRI
(Environment Canada 2002b). For 1999, Canadian total on-site releases of various phthalates were Bis(2-
ethylhexyl) phthalate 10.285 tonnes, Butyl benzyl phthalate 10.844, Dibutyl phthalate 1.409 tonnes, and Di-n-
octyl phthalate 0.200 tonnes (Environment Canada 2001b).
2.2.4.10 Nonyl- and Octyl-phenols
Source: NPEs and their degradation products (e.g., nonylphenol [NP]) are not produced naturally. Their
presence in the environment is solely a consequence of anthropogenic activity. NP and NPEs enter the
environment primarily via industrial effluents and municipal wastewater treatment plant effluents (liquid and
sludge), but also by direct discharge, although it is not known how significant the latter pathway is in Canada
(Health Canada 2001). Nonylphenol ethoxylates (NPEs) are a class of the broader group of compounds known
as alkylphenol ethoxylates (APEs). Once NPEs are released to sewage treatment systems, several
transformations can occur. Under aerobic and anaerobic treatment conditions, biodegradation to more toxic (and
estrogenic) metabolites occurs. These products are NP, nonylphenol ethoxylate (NP1EO), nonylphenol
diethoxylate (NP2EO), nonylphenoxyacetic acid (NP1EC) and nonylphenoxyethoxyacetic acid (NP2EC).
The amount of NP plus NPEs available for use in Canada (domestic production plus imports minus exports) was
23,800 and 19,000 tonnes in 1995 and 1996, respectively. It is not known how much of those totals refer to NP
and how much to NPEs. NPEs were manufactured at three facilities in Canada in 1995 and 1996 (Environment
Canada, 2001).
As reported in surveys of Canadian industry carried out under authority of Section 16 of CEPA, the total amount
of NP used by industry in Canada in 1996 was 5000 tonnes, with the majority being used as chemical
intermediates. The total reported use of NPEs in the same year was also 5000 tonnes (Environment Canada,
2001).
In 1989, domestic demand for NP in Canada was 4500 tonnes: 3700 tonnes were from domestic production,
1800 tonnes were imported and 1000 tonnes were exported. In that year, 1000 tonnes were used in ethoxylated
textile specialties, 1600 tonnes in ethoxylated pulp mill specialties, 500 tonnes in miscellaneous ethoxylates, 500
tonnes for TNPP and 900 tonnes for miscellaneous uses, including pesticides and lube oil (Environment Canada,
2001.
Although forecasts for demand of NP and NPEs in Canada were not available, the growth for NP in the United
States was 2% per year in the period 19881997 and was forecast at 12% per year from 1998 through 2002
(Environment Canada, 2001).
Use: NPEs are high-volume chemicals that have been used for more than 40 years as detergents, emulsifiers,
wetting agents and dispersing agents. Nonylphenol polyethoxylate-containing products are used in many sectors,
including textile processing, pulp and paper processing, paints, resins and protective coatings, oil and gas
recovery, steel manufacturing, pest control products and power generation (Environment Canada, 2001).
A variety of cleaning products, degreasers and detergents are also available for institutional and domestic use.
These products have numerous applications, including controlling deposits on machinery, cleaning equipment,
scouring fibres, as wetting and de-wetting agents, in dyeing, in machine felt cleaning and conditioning and in
product finishing. NPEs are also used in a wide range of consumer products, including cosmetics, cleaners and
paints, and in a variety of applications (Environment Canada, 2001).
41
Emissions: U.S. industrial emissions of nonyl- and octylphenols are not inventoried by the TRI (U.S. EPA
2002a).
For 1999, Canadian total on-site releases were: nonylphenol 0.661 tonnes, nonylphenol ethoxylates 193.624
tonnes (Environment Canada 2001b). For 2000, total on-site releases reported under the NPRI were:
nonylphenol 0.93 tonnes and nonylphenol ethoxylates 150.20 tonnes (Environment Canada 2002c).
Canadian industrial emissions of octylphenol are inventoried by the NPRI (Environment Canada 2002b). There
were no on-site releases reported under NPRI for OP in either 1999 or 2000 (Environment Canada 2002c).
Environment Canada has proposed changes and additions to the NPRI to ensure a more accurate accounting of
NP, NPE, OP and OPE releases to the environment.
2.2.4.11 Atrazine
Source: Atrazine is produced by a continuous process where isopropylamine is reacted with cyanuric acid under
basic conditions, forming 2,4-dichloro-6-isopropylamino- s-triazine, which is then reacted with monoethylamine
and dilute caustic to form atrazine. The approach allows for continuous product recovery, solvent recycling, and
waste removal. The triazine herbicides were first synthesized in 1955 and atrazine was first registered for use by
the Ciba-Geigy Corporation in 1958. It has been used over the last 40 years as an effective broad-leaf herbicide
in corn, sorghum, and sugar cane, and has also been used for other crops and for non-specific treatment of weeds
along railway right of ways and highways. Some of the latter uses have been curtailed to lessen atrazine release
into surface waters (ATSDR 2002).
Atrazine is designated as a restricted use pesticide (RUP), and is not available to the general public. RUPs are,
by law, for retail sale to, and for use by, only certified applicators or persons under their direct supervision, and
only for those purposes covered by the applicator's certification. Atrazine received this classification on January
23, 1990. Current trade names for atrazine include Aatrex®, Atranex, Atred, Gesaprim®, Primatol, and Vectal.
Atrazine is available in different formulations, including suspension concentrates, wettable powders, flowable
liquids, and water-dispersible granules (ATSDR 2002).
There are 21 facilities that manufacture or process atrazine in the U.S. The amounts manufactured or processed
range from 100 to 1,000 pounds in Alabama, Illinois, and Louisiana to very large formulation activities
(1,000,0009,999,999 pounds) in Mississippi, Missouri, and Nebraska. Facilities in Alabama and Iowa also
process atrazine in large amounts (up to 9,999,999 pounds), but Louisiana houses facilities that process the
greatest amounts of atrazine (up to 49,999,999 pounds), with activities including production, processing,
formulation, repackaging, sale and distribution, and other ancillary uses (ATSDR 2002).
There are six U.S. companies that are registered to produce products containing atrazine. Most of these
companies produce a technical-grade atrazine, with a purity ranging from 95.2 to 97%, although higher purity
atrazine can be produced (>99%). The technical-grade compound may contain three classes of impurities,
namely dichlorotriazines, hydroxytriazines, and tris(alkyl)aminotriazines. These impurities have not been
quantified in the available literature (ATSDR 2002).
Use: Numerous herbicides are registered for broadleaf and/or annual grass control applied as preplant
incorporated (PPI), pre-emergence (PRE) or early post-emergence (EPOST) treatments in field corn grown in
Eastern Canada. Products containing atrazine are registered for use in field corn in Canada. Also, atrazine is
registered for use in triazine-tolerant canola (TTC) grown in Eastern Canada (Pest Management Regulatory
Agency 1996).
Atrazine is the most heavily used pre- and post-emergence herbicide in the United States. It is used for the
control of grasses and broad-leafed weeds, and is primarily used on corn, sorghum, sugarcane, macadamia nuts,
and conifer tree crops; over 65% of the corn crop acreage in the United States is treated with atrazine. Atrazine
has been used in this capacity as a broad leaf herbicide for the last 35 years. It should be used at the appropriate
application rates, which have been reduced to 1.42.0 pounds per acre. The U.S. EPA has estimated that 3135
million kg of active ingredient atrazine were used on agricultural crops in the years 1987, 1993, and 1995
(ATSDR 2002).
42
More specific information is available from a National Center for Food and Agricultural Policy document that
reported trends in pesticide use between 1992 and 1997. Atrazine use showed a slight (3%) increase in use from
1992 to 1997. In 1992, 73,315,295 pounds (33 million kg) were used, and in 1997, 74,560,407 pounds (34
million kg) were used. Corn and sugarcane crops received significant increases in atrazine treatment in 1997 as
compared to 1992; sugarcane crops received 503,000 more pounds and corn crops received 2,037,000 more
pounds. Sorghum crops, in contrast, were treated with 1,065,000 pounds less in 1997 as compared to 1992.
This, however, was likely related to much less sorghum being planted in 1997 as compared to 1992. It should
be noted, however, that in some areas, corn growers decided to replace atrazine pre- and post-treatments with
other products. This decision was a result of restrictions placed on the use of atrazine, such that the application
rate restrictions reduced effectiveness on certain weeds (ATSDR 2002).
Atrazine usage rates have been relatively constant since monitoring began, but are beginning to decrease. In
1993, 4,955,300 pounds (2,247,093 kg) of atrazine were used on 45,333,000 acres (18,346,014 hectares) of corn
in the United States; the maximum reported usage was in 1976, when 9,034,000 pounds (4,097,796 kg) of
atrazine were used in all agricultural applications. Of that, 8,379,000 pounds (3,800,689 kg) were applied to
corn. Also in 1976, the largest number of acres was treated with atrazine, with 61,750,000 total acres
(24,989,883 hectares) being treated. More than 92% of the total acreage treated with atrazine (56,863,000 acres;
23,012,141 hectares) was corn crops. Atrazine is a restricted use pesticide and is only available to applicators
who meet appropriate requirements of the state and federal government (ATSDR 2002).
In Canada, as a result of a re-evaluation announced in 1988, significant changes to the allowable uses of atrazine
were made: the use rate in corn was cut by two thirds and the highest-rate uses (industrial uses, such as on
parking lots) were eliminated. Buffer zones were implemented to protect aquatic habitat.
Currently, the only remaining major use of atrazine in Canada is on corn. The total amount of atrazine used in
Canada is 1.5% of the amount used in the US.
Emissions: U.S. industrial emissions of atrazine inventoried by the TRI for the 2000 reporting year are as
follows (U.S. EPA 2002b):
Total on-site releases were 536,771 pounds (of these, releases to air were 33,807 pounds, releases to water were
1,034 pounds, releases via underground injection were 198 pounds, and releases to land were 501,732 pounds)
and total off-site releases (transfers to disposal) were 74,734 pounds. Total on- and off-site releases were
611,505 pounds.
Canadian industrial emissions of atrazine are not inventoried by the NPRI (Environment Canada 2002b).
Atrazine is one of the primary herbicides currently used in the Great Lakes region.
Approximately 2,790 kg (6,151 lb) enter the lake each year through the atmosphere (LaMP 2000).
2.2.4.12 Perfluorooctane Sulfonate (PFOS)
Source: Perfluorooctane sulfonate (PFOS) is a member of a large family of perflurooctanyl sulfonate chemicals.
It is common to refer to perfluorooctane sulfonate (PFOS) and its salts as simply, PFOS (OECD 2002a, OECD
2002b). These chemicals have been used in a variety of industrial, commercial and consumer products in
Australia (NICNAS 2002). Based on data on PFOS the U.S., EPA believes that this substance and other
structurally related substances may persist in the environment, bioaccumulate, and be toxic (U.S. EPA 2002d).
The recent reports from the OECD 34th Joint Meeting of the Chemicals Committee and the Working Party on
Chemicals, Pesticides and Biotechnology (5-8 November 2002), conclude that "PFOS is persistent,
bioaccumulative and toxic to mammalian species." (OECD 2002a)
The 3M Company was the largest worldwide producer of PFOS chemicals. On May 16, 2000, 3M announced its
decision to phase-out the manufacture of all products based on perfluorooctanyl chemistry. In this
announcement, 3M indicated that its worldwide commercial production of these materials would be
discontinued substantially by the end of 2000 and eliminated completely by the end of 2002. 3M has stated that
its phase-out decision was based on its commitment to responsible environmental management and sound
business principles. Global production from all sources has been about six and a quarter of a million kilograms
per year of 25 different compounds. According to the OECD, the number of production sites is not clear, but
43
there is production in the US, Europe and Japan of approximately 4,500 metric tons of PFOS-related chemicals
annually (OECD 2002b). Paper protection alone uses more than a million kilograms per year. The losses from
production to the environment are significant. Annual releases are estimated to be 39,000 kilograms into air,
500,000 kilograms into streams, and 4,600 kilograms to wastewater. PFOS does not biodegrade in the
environment (NICNAS 2002).
Use: PFOS chemicals are used in carpet cleaners, cement additives, antifogging agents, fire-fighting foams,
adhesives, pesticide formulations, and paper coatings. Until its phase out, the 3M Company (USA) had been
producing most of the electrochemically fluorinated surfactants since 1949. PFOS has been a component of the
ScotchgardTM range of products and is an ingredient of some industrial additives and fire fighting foams
produced by the 3M Company. The fire fighting foams are used to extinguish Class B fires that involve
flammable fuels. ScotchbanTM was a PFOS-containing product supplied by 3M to treat paper containers for fast
and pet food (NICNAS 2002).
Emissions: U.S. industrial emissions of PFOS are not inventoried by the TRI (U.S. EPA 2002b).
Canadian industrial emissions of PFOS are not inventoried by the NPRI (Environment Canada 2002b).
2.2.5 Organometals
The main constrain in better understanding the sources, fate, and a biogeochemical cycle for organometalic
compounds is lack of consistent analytical procedures. Limited number (>5) of laboratories in Canada and US
are capable of producing reliable data in this field. Currently there is no capacity in Mexico for these types of
measurements.
2.2.5.1Organotin (Triorganostannic) Compounds
Source: Tin is a natural element in the earth's crust. When combined with chlorine, sulphur, or oxygen, it is
called an inorganic tin compound. These compounds are not of interest in this assessment. When tin is combined
with materials that contain carbon, it is called an organotin compound. Methyltin compounds are primarily
produced by microorganisms.
TBT is extremely toxic to aquatic organisms, and is considered an endocrine disrupting compound. TBT has
high Kow values and has tendency to bioaccumulate in the food web. TBT contamination appears to be wide
spread particularly in industrialized estuaries and naval shipyard.
Organostannic compounds particularly methylated tin compounds have been observed in natural waters, marine,
and lacustrine sediments though environmental conditions have not been well established (Pelletier 1995).
From January to August 2002, Mexico imported 28 tonnes of organotin compounds from the US (Economic
Secretariat).
Use: Organostannic compounds have been used as stabilizers (mono- and di- compounds), wood and materials
preservatives (tri- compounds), curing agents (mono- and di- compounds), catalysts (mono-and di- compounds)
in silicone and plastics. Tribtutyltin is primarily used as an antifouling pesticide. Its use is generally prohibited
on vessels >25m, and is expected to be banned in antifouling paints by 2008.
Emissions: Although U.S. industrial emissions of organotin compounds in general are not inventoried by the
TRI (U.S. EPA 2002a), certain triorganostannic pesticides are; Canadian industrial emissions of organostannic
compounds are not inventoried by the NPRI (Environment Canada 2002b).
2.2.5.2 Organomercury Compounds
Source: It is a precious metal used in chlor-alkali production, wiring devices, switching mechanisms, amalgam
dental fillings, and measurement and control instruments. Industries also manufacture and process mercury
reagents, catalysts, and medicinal chemicals. Metal ores, coal, crude oil, and fuel oils contain mercury as a trace
constituent (U.S. EPA 2002a).
Mercury combines with carbon to make organic mercury compounds, the most common being methylmercury
(MeHg). Methylmercury has no industrial uses; it is formed in the environment from the methylation of the
inorganic mercurial ion (U.S. EPA 2002a).
44
Methylmercury is the most toxic form of mercury, and also a known neurotoxin. The development of neural
tissue in mammals and birds are severely affected by methylmercury. Methylmercy bioaccumulates in fish and
shellfish, in fact more than 90% of the mercury detected in biota is in the form of methylmercury. Fish
consumption is the major source of methylmercury, of particular concern is the exposure of fetus and young
children to high levels of this compound. The main source of methylmercury in the environment is from the
direct methylation of inorganic mercury by methanogenic bacterium in water, soil and sediments. High levels of
methylmercury (commonly referred to as mercury) in fish are the main source of the fisheries closers in North
America.
Methylation and demethylation of mercury plays an important role in the biogeochemical cycle of mercury.
Persistent and fate of methylmercury: Methylmercury speciation in the aquatic environment is strongly
dependent upon pH and chloride concentration. The biological half-life of methylmercury in aquatic organisms
such as fish and crustaceans is in order of several hundred years.
Mercury is present in air, water and soil from both natural sources and as a result of human activity.
All soils in Canada contain mercury at levels averaging about 0.06 µg/g. Higher natural background
contamination is associated with rock and mineral deposits in the Canadian Shield and in the Rocky Mountains.
Weathering of naturally occurring mercury in rocks and industrial effluent are the major sources of water
contamination. Water sources near mercury deposits have been found to contain up to 80 µg mercury/L,
compared with 0.1 µg/L in low natural mercury areas (Health Canada 1998). Mercury and methylmercury
concentrations in water of pristine lakes and rivers are usually below 10ng/L and 1ng/L respectively.
Major sources of recycled or recovered mercury include scrap from instrument and electrical manufactures
(lamps and switches), wastes and sludge from laboratories and electrolytic refining plants, mercury batteries,
and dental amalgams (U.S. EPA 2002a).
Anthropogenic sources in Canada include historical industrial activity (old mercury, gold, and silver mines and
their tailings); releases from sulphide ore (copper, lead, nickel) smelting activities, coal-burning plants, and the
burning of other fossil fuels; chlor-alkali plants; historical use of mercury as a slimicide in the pulp and paper
industry; and the historical agricultural use of alkyl mercury fungicides. Registration for the treatment of cereal
grains with alkyl mercury fungicides was discontinued in Canada in 1973. The amount of mercury released into
the environment has decreased steadily since the early 1970s due to regulations and changes in industrial
processes. Mercury has not been mined in Canada since 1975 (Health Canada 1998).
Use: Mercury imports to Canada have fallen from 40-50 tonnes in 1985 to about six tonnes in 1995. Canada is a
net exporter of mercury. The Canadian trade in mercury cell batteries has decreased from about three million
units in 1990 to 0.7 million units in 1995, a 76 percent reduction. Mercury consumption in Canada is attributed
mainly to two sectors, electrical apparatus, including control instruments, and the one remaining mercury cell
chlor-alkali plant. Mercury consumption in Canada was 6 tonnes in 1994 and was further reduced to 2.9 tonnes
in 1995. Anthropogenic emissions of mercury to the atmosphere dropped from 39 tonnes in 1990 to about 20
tonnes in 1995, a 49 percent reduction (CEC 1998).
There are many additional uses of mercury including dental amalgam; thermometers, electrical switches, and
batteries; and paint preservatives. Although used in the past, mercury is no longer used in medicine (diuretics,
antiseptics, and skin preparations). Prior to 1991, 30 percent of interior latex paint contained mercury
compounds as a preservative. This was discontinued in January 1991 for interior latex paints but exterior paints
may still contain mercury. Mercury salts are also used in skin-lightening creams and as antiseptic creams and
ointments (Health Canada 1998).
In the U.S., mercury has been eliminated from paint manufacturing since 1994. Mercury in batteries declined
from 106 tonnes in 1990 to just 6 tonnes in 1994. (CEC 1998). The battery industry eliminated its use of
mercury in all but button cells by 1993. Button cells sold in the US in 2000 used 2 tons of mercury.
("Household Batteries and the Environment" at www.nema.org/batteryehs .)
Overall, domestic U.S. consumption of mercury has shown a downward trend since the early 1970s. In 1995,
consumption was 463 metric tons, down 10% from 1994. The largest commercial use of mercury in the United
45
States was for electrolytic production of chlorine and caustic soda in mercury cells, accounting for 35% of
domestic consumption. Manufacture of wiring devices and switches accounted for 19%, measuring and control
instruments for 9%, dental equipment and supplies used 7%, electric lighting used 7%, and other uses used 21%.
Due to the high toxicity of mercury in most of its forms, many applications have been cancelled as a result of
attempts to limit the amount of exposure to mercury waste (ATSDR 2002).
In Canada, industry-led voluntary initiatives, together with federal and provincial regulations, have resulted in
significant declines of mercury in products and mercury emitted to the atmosphere.
The Economic Secretariat reported that from January to August this year Mexico has imported 127.62 tonnes of
this material (98% from the U.K.). According to the Authorized Pesticides Guide for Agriculture, released by
the Office for Crops Health, Mexico has prohibited the import, manufacture, formulation, commercialization,
and usage of mercuric pesticides.
Emissions:
Atmospheric mercury is primarily a result of global off-gassing of mercury from soils and surface waters and
anthropogenic emissions from the burning of fossil fuels, medical waste incineration, municipal waste
combustion and chlorine production. In the USA, the latter four sources account for over 70% of anthropogenic
emissions, which were reported at 187 short tons in 1996. (US EPA 2001) This number has been revised
downward as a result of improved calculations. Additional significant reductions will be registered in the next
progress report (for the year 1999) when the emission reductions strategies particularly for medical waste
incineration and municipal waste combustion will reduce the total by a further estimated 35 short tons.
In 1994-1995, mercury emissions from "area sources" (i.e., general emissions rather than a specific fixed
source), totalled 3.4 tons (7,500 pounds). More than half of these emissions were from lamp breakage and
general laboratory use. Other "area sources" in 1994-1995 included dental preparations, landfills, mobile
sources, paint use, and agricultural burning (U.S. EPA 2002a).
U.S. industrial emissions of mercury and mercury compounds inventoried by the TRI for the 2000 reporting
year are as follows (U.S. EPA 2002a):
Total on-site releases were 3,466,789.83 pounds (of these, releases to air were 164,492.53 pounds, releases to
water were 2,302.28 pounds, underground injection were 11,713.52 pounds, and on-site releases to land were
3,288,281.49 pounds) and total off-site transfers to disposal were 849,872.31 pounds. Total on- and off-site
releases were 4,316,662.14 pounds.
Total U.S. mercury and mercury compounds in industrial production-related waste were projected to decline by
8.5 percent by 2002 (U.S. EPA 2002a).
Canadian anthropogenic emissions of mercury to the atmosphere dropped from 35 tonnes in 1990 to about 12
tonnes in 1995, a 65 percent reduction and to 8 tonnes for 2000 (Environment Canada, Pollution Data Branch,
Personal Communication, Oct 09, 2002).
Canadian industrial mercury and mercury compound emissions were inventoried by the NPRI for the 2000
reporting year and are available through the Query Site (NPRI Query 2002). At the time of the writing of this
report, no formal report had been published by the NPRI (Environment Canada 2002b). For 1999, Canadian
total on-site releases of mercury and mercury compounds were 3.608 tonnes (Environment Canada 2001b).
It is estimated that mercury emissions in Mexico reach 31.5 ton/year. This calculation is based in annual
productivity of industrial sources, commonly accepted emission factors and information available for mercury
content in products and processes. The most important industrial and non industrial sources include mining,
foundries, chlor-alkali cell manufacture, petroleum refining, and diverse usage in manufacture of thermometers,
electrical switches and fluorescent lamps.
2.2.5.3 Organolead Compounds
Source: Although lead occurs naturally, its dispersion throughout the environment is mostly due to human
activities. Lead-acid battery waste is the most common source of lead, although most batteries are now recycled.
Other uses include gasoline, the production of ammunition, metal products, paints, ceramic glazing, mining,
46
smelting, refining of lead ores and the secondary smelting of lead containing materials (these are also sources
for other metal emissions).
There are three major sources of organolead compounds in the environment:
a) Antiknocking agents: antiknocking agents in fuels were introduced in 1923 in the US. Both trimethyl and
tetraethyl lead have been used as anitknocking agents. Even though the majority of the alkylead compounds
degraded during the combustion process a small percentage of the material was released as the result of
incomplete combustion, spillage, and direct evaporation. Tetraethyllead was also released from the
manufacturing plants. The use of leaded fuels was the primary source of lead in the atmosphere, however,
concentrations in the air have declined significantly since the introduction of unleaded gasoline in 1975
(Health Canada 1998).
b) Trialkyllead compounds have also been used in a number industrial application including alkyaltion
processes, polymerization reactions, stabilizers in PVC, and biocides.
c) Direct methylation of inorganic lead is the third source organolead compounds in the environment. This
process is not well understood.
In Canada, use of lead in fuels was banned after 1990, with a few minor exemptions. In 1976, the Hazardous
Products Act regulated lead levels for paint used in interiors. Although the lead content of exterior paint is not
regulated, a voluntary agreement with Canadian paint manufacturers ensures that no lead will be intentionally
added to exterior paint (Health Canada 1998).
In the early 1970s, the U.S. EPA set national regulations to gradually reduce the lead content in gasoline. In
1975, unleaded gasoline was introduced for motor vehicles equipped with catalytic converters. The U.S. EPA
banned the use of leaded gasoline in highway vehicles in December 1995 (U.S. EPA 2000a).
Domestic U.S. lead metal production rose at an annual rate of 1.3% between 1990 and 1996, going from 1.33
million metric tons to a record high of 1.43 million metric tons. Primary lead production declined at an annual
average rate of 3.2% during this time period, dropping from 404,000 metric tons in 1990 to 326,000 metric tons
in 1996. This decline was a result of cutbacks in production in 1991 and 1992 in response to low lead prices and
of the closure of the primary lead refinery in Nebraska in 1996. Primary lead production increased to 343,000
metric tons in 1997. Secondary lead production, however, rose at an average annual rate of 3.2%, climbing from
922,000 metric tons in 1990 to 1.1 million metric tons in 1996 and 1997 as the closure of 5 small secondary
refineries was more than offset by the opening of a new secondary refinery and an increase of capacity at a
number of other secondary facilities. As a result, secondary lead's share of total lead metal production rose from
69.5% in 1990 to 77.1% in 1996. (ATSDR 2002).
Use: Lead may be used in the form of metal, either pure or alloyed with other metals, or as chemical
compounds. The commercial importance of lead is based on its ease of casting, high density, low melting point,
low strength, ease of fabrication, acid resistance, electrochemical reaction with sulphuric acid, and chemical
stability in air, water, and soil. At least half of all lead consumed worldwide goes into producing lead-acid
batteries used in automotive and various industrial applications. Certain dispersive or readily bio-available uses,
such as lead in gasoline, as a solder in piping for drinking water and food cans, and in house paints, have been or
are being phased out due to environmental and health concerns (ATSDR 2002).
Reported consumption of lead in the U.S. increased at an average annual rate of 3.3% between 1990 and 1996.
Consumption patterns have long been shifting to a market dominated by one major end use: the lead-acid
battery. Increasing lead-acid battery demand has more than made up for all end-uses that have either
significantly declined or been legislated out of existence for environmental and health reasons. The lead-acid
battery share of total domestic lead consumption increased from 79.7% in 1990 to 87.6% in 1996 and grew at an
average annual rate of 5.2% over the period. At the same time, non-battery uses of lead declined at an average
annual rate of 4.5%. Except for a sharp increase in 1995, lead used in ammunition (the largest non-battery end-
use) remained fairly constant during this period. Other uses, such as cable covering, caulking, and solder, have
declined significantly while tetraethyl lead additives for gasoline, which once accounted for 20% of domestic
consumption, has been phased out except for the exceptions noted above (ATSDR 2002).
47
Emissions: U.S. industrial emissions of lead and lead compounds inventoried by the TRI) for the 2000 reporting
year are as follows (U.S. EPA 2002b):
Total on-site releases were 349,941,075 pounds (of these, releases to air were 1,485,834 pounds, releases to
water were 95,089 pounds, underground injection were 8,572,841 pounds, and on-site releases to land were
339,787,312 pounds) and total off-site transfers to disposal were 37,288,872 pounds. Total On- and Off-site
releases were 387,229,948 pounds.
Although Canadian industrial lead and lead compound emissions were inventoried by the NPRI for the 2000
reporting year, at the time of the writing of this report, no formal report had been published by the NPRI
(Environment Canada 2002b). For 1999, Canadian total on-site releases of lead and lead compounds were
3,495.282 tonnes (Environment Canada 2001b).
2.2.6 Critical Considerations Concerning PTSs in Mexico
During the analysis of the information that could be compiled, with regard to PTSs handling in Mexico, the
following critical points were identified.
Environmental Management
Apparently, formal users are well aware of the measures to be taken in the case of PTS intoxication. However,
they are not aware of the appropriate procedures to be followed in cases of environmental pollution where PTSs
are detected in the air, water or soil. In this case, there may be PTSs in the environment without having been
detected, since there is no monitoring program for these substances or the infrastructure for the environmental
authorities to do so. In the case of detection, there is no standard procedure to handle the problem.
Emergency Management
Given the possible risk of accidents involving a PTS, citizen protection actions must be taken. In this regard, the
national civil protection system is responsible for handling technological events, including those involving
chemical substances. There is a high degree of risk for industries and distributors, as well as in businesses
carrying on high-risk activities. There is also a high risk of occurrence of events of this nature in the transport of
PTSs. The federal preventive police also participate in highway surveillance, although such personnel are not
trained for handling this type of event; this is certainly the case also for the municipal fire corps.
Training
We found that some of the existing shortcomings in PTS handling give rise to the need to establish training
programs, not only geared toward teaching proper handling practices and exposure and occupational risk
reduction measures, but also for training users and personnel involved in handling the environmental risks or
catastrophes caused by accidents. Such training will require the development of procedural manuals, considering
that the persons responsible for such programs do not have a specialized technical background.
Monitoring and Oversight
Although the environmental monitoring techniques for PTS detection are widely known, there are no
environmental monitoring programs to improve the handling thereof even though this type of network is
indispensable.
Collection and Disposal of Packaging
The abandonment of empty packaging for agrochemicals represents a problem, in that it carries the risk of
environmental releases and health risks for individuals who reuse them for purposes far from their intended use.
Despite the AMIFAC's National Program for the Collection of Empty Fertilizer Packaging called "Keep the
Field Clean," lax oversight has impeded the attainment of the expected results.
The objectives of the AMIFAC program include: eliminating the practice of container reuse; promoting the
optimal usage of agrochemical products; avoiding pollution of aquifers and irrigation channels; collecting
containers from the field; establishing handling criteria; and reasonably and safely destroying the containers. In
2000, AMIFAC, through its distributors, destroyed approximately 60 tons of packaging through heat treatment.
Although this program was well conceived, there is no standard to support it and it does not apply penalties,
being based solely on user participation without any oversight to ensure compliance.
48
Agricultural Runoff Water
Considering that the use of PTSs relates heavily to agricultural activities, and that water usage in such activities
represents the greatest percentage of water consumption in general, there is a high probability that agricultural
runoff waters contain considerable levels of toxic substances. In some cases, farm networks carry wastewater to
the sea; given the persistent nature of the substances, the pollution is carried to places unknown determined by
the tide currents, affecting marine life.
The National Water Commission (Comisión Nacional del Agua--CNA) does not have the infrastructure needed
to monitor this type of pollution, known as non-point source pollution. The CNA laboratory network performs
sampling at a number of points, principally at bodies of water used as a source of drinking water. The
corresponding NOM requires laboratory analysis to detect the presence of persistent toxic substances and make
the appropriate decisions. Unfortunately, the CNA's capacity allows it only to assess the most elemental
parameters of the standard.
Illegal PTS Sales
Though their production, sale and use are banned, it is known that some products are sold in small
establishments, principally in urban areas where information on usage and container disposal is least accessible.
There are even cases where pesticides are applied directly on the bodies of persons unaware of the danger of
such practices.
Given this situation, the detection of the illegal product distribution is indispensable, requiring public awareness
for protecting people in their homes. Information should be publicized on the risks of poisoning and parent and
institutional meetings should be held; block captains should be trained to publicize these topics.
2.3 Hot Spots
Throughout North America chemicals have accumulated over time at intentional or unintentional sites. These
sites have become more and more important as the consequences of PTSs are being better defined and the
significance of these old sites, as current sources, is becoming clear. The chemicals at these sites, produces years
or decade ago, are being release and circulated again in the environment. Some of the major sites, called hot
spots, are discussed here.
Great Lakes Basin
The Great Lakes basin as a whole might be considered to be a hot spot (WWF 2000). Canada and the United
States have taken sweeping measures to address the pollution problems in the basin. The Great Lakes Remedial
Action Plan (RAP) program originated from a 1985 recommendation from the International Joint Commission's
Great Lakes Water Quality Board. The recommendation was formalized in the 1987 amendment to the Canada-
United States Great Lakes Water Quality Agreement under Annex 2 (Remedial Action Plans (RAPs) and
Lakewide Management Plans). The mission of the RAPs is to restore beneficial uses, both ecological and
cultural as identified in Annex 2, in degraded areas within the basin (Environment Canada 1999).
Significant progress has been made since the signing of the Great Lakes Binational Toxics Strategy (GLBTS) by
Canada and the United States in 1997. Both parties have engaged in a wide range of activities to address sources
of "Level I" substances which have immediate priority for virtual elimination. These substances include
mercury, polychlorinated biphenyls (PCBs), dioxins and furans, hexachlorobenzene (HCB) and benzo(a)pyrene
(B(a)P), octachlorostyrene (OCS), alkyl-lead, and five cancelled pesticides, namely, chlordane, aldrin/dieldrin,
DDT, mirex, and toxaphene. Achievements over the past five years are summarized in the GLBTS 5-Year
perspective published in May 2002 (http://www.binational.net/bns/menu-e.html).
Despite the success of the GLBTS program, problem areas still exist. Forty-three "Areas of Concern" (AOCs)
have been identified: 26 located entirely within the United States; 12 located wholly within Canada; and five
that are shared by both countries (U.S.EPA 2002e). Of the 12 AOCs located wholly within Canada, 11 are still
on the list (Environment Canada 1999). RAPs are being developed for each of these AOCs to address
impairments to any one of 14 beneficial uses (e.g., restrictions on fish and wildlife consumption, dredging
activities, or drinking water consumption) associated with these areas. Sediments have been identified as serious
49
problems in many AOCs. AOC Principles and Guidelines have been finalized for formally delisting these areas
as beneficial uses are restored (U.S. EPA 2002e).
U.S. EPA Superfund (CERCLA)
On December 11, 1980, United States Congress passed the Comprehensive Environmental Response,
Compensation, and Liability Act (CERCLA or Superfund). It is a comprehensive program to address abandoned
hazardous waste sites and other releases of hazardous substances (such as PAHs, PCPs, lead, mercury, and
dioxins). This important legislation was enacted to fill a major gap in environmental protection. The events at
Love Canal, New York, and other sites around the United States had shown that wastes buried long ago, and
mostly forgotten, could prove to be a serious threat to the community. The site that prompted Congress to enact
the Superfund legislation (Love Canal) has been cleaned (U.S. EPA 2000b).
The law authorizes two kinds of response actions: short-term removals, where actions may be taken to address
releases or threatened releases requiring prompt response and long-term remedial response actions, that
permanently and significantly reduce the dangers associated with releases or threats of releases of hazardous
substances that are serious, but not immediately life threatening. These actions can be conducted only at sites
listed on EPA's National Priorities List (NPL). A list of sites can be found on the U.S. EPA Superfund website
(U.S. EPA 2002e).
Superfund has produced the following results (U.S. EPA 2000b):
· Over 6,400 actions to immediately reduce threats to public health and environment.
· 757 Superfund sites with all cleanup construction completed.
· Of the 1,450 final NPL sites: 219 are deleted; and over 1,200 have all final cleanup plans approved.
· Of the 59 sites proposed for listing on the NPL, 28 have had, or are undergoing, some cleanup.
· Over 650 Five-Year Reviews have been completed to ensure long-term effectiveness of cleanup remedies.
Abandoned waste sites are still being discovered. EPA continues to work with its partners to address immediate,
or long-term, danger (U.S. EPA 2000b).
Nova Scotia: Sydney Tar Ponds
The Sydney Tar Ponds and Coke Ovens site is one of the largest and most hazardous chemical waste sites in
eastern Canada. More than 80 years of discharges from the coke ovens of an adjacent steel plant have
contaminated the 51 acre (23 hectare) site and the adjoining Muggah Creek.
By 1983, Environment Canada had pinpointed the coke ovens as the major source for pollution in the Muggah
Creek. Subsequent studies found that the air downwind of the coke ovens had elevated levels of polynuclear
aromatic hydrocarbon (PAH) contamination as well. In the early 1980s, a series of federal and provincial studies
revealed the presence of contaminants, especially PAHs and heavy metals, in the Sydney waterways. Scientists
from the Federal Department of Fisheries and Oceans also discovered levels of PAHs in lobsters high enough to
warrant indefinite closure of the commercial lobster fishery in the South Arm of Sydney Harbour (Ellicot 2002).
A detailed study of the whole area was undertaken in 2000-2001 to acquire more specific information about the
Sydney Tar Ponds and the surroundings.
· The North and South Tar Ponds contain an estimated 550,000 m3 of sediments contaminated with PAHs of
which 44,500 m3 are also contaminated with PCBs in concentrations over 50 parts per million.
· The sediments also contain heavy metals and other organic compounds.
· Surface soils in the area contain heavy metals such as beryllium, copper, zinc vanadium, arsenic, chromium
and thallium at levels above provincial/federal (CCME) commercial/industrial guidelines.
· PAHs are present in sub-surface soils and levels of Benzo(a)pyrene above provincial/federal (CCME)
commercial/industrial guidelines were found in some soil samples.
· PAH and heavy metal concentrations in groundwater across the east shoreline area are likely related to in-
fill material and adjacent historical land uses.
· Soil gas surveys in the Southeast Shoreline area showed hydrocarbon gases in soil although the Tar Ponds
were not identified as a source of this contamination.
50
2.4 Data Gaps and Programme Needs
2.4.1 Data Gaps
Mexican databases should be available in electronic media for easy and understandable public consultation. This
should apply not only to databases, but also to summaries and statistics which can be understood by any reader.
The national data bases should take account of extensive international systems guidelines for PTSs on such
items as definitions, identification, management, effects, risks, and documenting of these chemicals.
CICOPLAFEST could be charged with this activity as part of its fundamental actions.
The quality and adequacy of current Mexican databases needs to be assessed using statistical procedures to
validate the information.
In Mexico, PTSs quality control programs to ensure the veracity of the information are required for both the
information generated by specialized laboratories and that contained in the electronic media.
Substances for which no emissions data could be found for either the U.S. or Canada are endrin,
dichlorodiphenyltrichloroethane (DDT), mirex, hexachlorocyclohexanes (HCH), chlordecone, endosulfan,
hexabromobiphenyl (HxBB), tin compounds and two polybrominated diphenyl ethers, Octa- and Penta-BDE. In
the case of Canada, most of the pesticides are not produced or registered for use and therefore not inventoried
U.S. industrial emissions of dieldrin and nonyl- and octyl-phenols are not inventoried by the federal government
[i.e., U.S. Toxics Release Inventory (TRI)] (U.S. EPA 2002a). Furthermore, there is a necessity for governments
to monitor the emissions of nonylphenol polyethoxylates (NPE) and octylphenol polyethoxylates (OPE) as well
as octylphenol and nonylphenol. The APEs (NPE & OPE) degrade during both aerobic and anaerobic processes
to form OP and NP, as degradation intermediates, as well as other potentially harmful substances, such as the
carboxylic acid degradation products. Consequently, monitoring just the releases of NP and OP will not present
an accurate picture of their potential for harm to the environment.
Canadian industrial emissions of aldrin, dieldrin, chlordane, heptachlor, pentachlorophenol (PCP), toxaphene
and polychlorinated biphenyls (PCBs) are not inventoried by the Canadian National Pollutant Release Inventory
(NPRI)] (Environment Canada 2002b). As noted above, most of the pesticides are not produced or registered for
use and therefore not inventoried.
There are various limitations associated with the federal industrial pollutant release and transfer inventories of
the U.S. (TRI) and Canada (NPRI). A principal factor in making good use of pollutant release and transfer data
from the Canadian (NPRI) and U.S. (TRI) inventories is to know their limitations (CEC 2002). The data:
· do not encompass all potentially harmful substances;
· do not address all sources from which chemicals of concern move into the environment;
· in some cases, do not identify all on-site releases and off-site transfers from a facility;
· do not measure releases and transfers-they estimate them;
· do not supply a direct perspective on the ultimate environmental fate of chemical substances that reporting
facilities release or ship off-site for disposal or other disposition;
· do not provide information on the toxicity or potential health effects of substances released or transferred by
reporting facilities;
· do not indicate risks from substances released or transferred by reporting facilities;
Other important information also lies beyond the bounds of these data. For example, information about
local/regional geography, demographics, and economics may be needed to interpret this data appropriately in
community and ecological contexts (CEC 2002).
2.4.2 Programme Needs
For the North American region, two major programme need occurs in Mexico.
Mexican information is found in the "Green Catalogue" and "Red Catalogue" kept by CICOPLAFEST. The
information in both catalogues is not easily accessible by the public, requiring specialized training to understand
them.
51
Users and members of civil organizations interested in environmental conservation and protection usually do not
have sufficient training to make use of existing databases.
2.5 Conclusions
The national substance inventories have become valuable databases. However, for a number of important
substances there are no national emissions databases in either the U.S. or Canada.
The national databases in Canada and the USA have intrinsic weaknesses, including,
· They do not address all sources;
· They do not identify all on-site releases and off-site transfers from a facility;
· Some releases are estimated not measured;
· They do not indicate the ultimate environmental fate of materials which porting facilities release or ship
materials off-site for disposal or other disposition,;
· They do not provide information on the toxicity or potential health effects of substances which reporting
facilities release or transfer.
· They do not identify exposure risk to human or ecological populations from substances released or
transferred by reporting facilities.
In Mexico, many required databases are limited or lacking. Access to information is difficult requiring intensive
searches to determine, when possible, the character, quantities and movements of PTSs.
CICOPLAFEST's efficient regulation and coordination of the handling and use of toxic substances has not been
achieved, due to the limited staffing of the Commission and the lack of administrative power. Agencies such as
the CNA--part of Semarnat--have no formal communication with the entities forming part of CICOPLAFEST.
There should be an easy-to-use information system to provide data and statistics on PTS handling, as well as
pollution prevention measures, health risks and individual emergencies and disasters. To help overcome this
situation, major agrochemical producers should perform assessment studies, particularly for those compounds
for which long range transport potential has not been determined and for those chemicals which pose
environmental and human health risks because of their mutagenic or carcinogenic properties.
Procedures and criteria should be applied to prioritize PTS management in Mexico, including various concepts
such as handling volumes, uses, health and environmental effects, etc. Unfortunately, there is insufficient
domestic information available to develop such procedures and criteria.
There are no statistical data showing the true extent of toxic substances entering Mexico. The issuance of import
permits should be clear, not just for importers but for the general public.
2.6 References
ATSDR 2002. Agency for Toxic Substances and Disease Registry http://www.atsdr.cdc.gov
Bailey, R. E. 2001. Global hexachlorobenzene emissions, Chemosphere, 43, 167-182.
Barrie, L.A., D. Gregor, B. Hargrave, R. Lake, D. Muir, R. Shearer, B. Tracey and T. Bidleman 1992. Arctic
contaminants: sources, occurrence and pathways. Sci. Total Environ. 122: 1-7
Barrie, L., R. Macdonald, T. Bidleman, M. Diamond, D. Gregor, R. Semkin, W. Strachan, M. Alaee, S. Backus,
M. Bewers, C. Gobeil, C. Halsall, J. Hoff, Y.F. Li, L. Lockhart, D. Mackay, Muir, D., J. Pudykiewicz, K.
Reimer, J. Smith, G. Stern, W. Schroeder, R. Wagemann, F. Wania, M. Yunker. Chapter 2. Sources, Occurrence
and Pathways, In: Jensen, J., Adare, K. and Shearer, R. (Eds), Canadian Arctic Contaminants Assessment
Report, Department of Indian and Northern Affairs, Ottawa 1997. pp. 25-182.
BSEF 2000. Bromine Science and Environmental Forum (BSEF), Belgium.
http://www.ebfrip.org/download/weeeqa.pdf
CACAR 2002. Canadian Arctic Contaminants Assessment Report (CACAR) - in preparpation. Department of
Indian and Northern Affairs, Canada.
52
CCC 2002. Chlorine Chemistry Council, Arlington, Virginia, USA.
http://c3.org/chlorine_issues/understanding_dioxin/dioxin_brochure/b-by-products-blue.html
CEC 1998. The Sound Management of Chemicals Initiative under the North American Agreement on
Environmental Cooperation: Regional Commitments and Action Plans, North American Commission for
Environmental Cooperation:
CEC 2002. Taking Stock 1999, North American Pollutant Releases and Transfers Sourcebook, North American
Commission for Environmental Cooperation.
CEPA 1990. Canadian Environmental Protection Act: Priority Substances List Assessment Report No. 1 -
Polychlorinated Dibenzodioxins and Polychlorinated Dibenzofurans. http://www.hc-sc.gc.ca/hecs-
sesc/exsd/cepa/dioxins_furans.pdf
CEPA 1993. Canadian Environmental Protection Act: Priority Substances List Assessment Report -
Hexachlorobenzene.http://www.hc-sc.gc.ca/hecs-sesc/exsd/cepa/hexachlorobenzene.pdf
CEPA 1994. Canadian Environmental Protection Act: Priority Substances List Assessment Report Polycyclic
Aromatic Hydrocarbons. http://www.hc-sc.gc.ca/hecs-sesc/exsd/cepa/polycyclic_aromatic_hydrocarbons.pdf
CEPA 1999. Canadian Environmental Protection Act: Priority Substances List Assessment Report Nonylphenol
and its Ethoxylates.http://www.ec.gc.ca/substances/ese/eng/psap/final/npe.cfm
CPIA 1990 update. Chlorinated Paraffins Industry Association: Chlorinated Paraffins: A Status Report.
CPIA 2001. Chlorinated Paraffins Industry Association: Chlorinated Paraffins: A Status Report.
http://www.regnet.com/cpia/status_report.html
Ellicot 2002. Ellicot Case Studies. http://www.dredge.com/casestudies/enviro8.htm
Environment Canada 1992. Agriculture Canada - Environment Canada Pesticide Registrant Survey Report 1988
Data, prepared by Commercial Chemicals Branch Conservation and Protection, Environment Canada.
Environment Canada 1999. Binational Remedial Action Plans (RAPs) website.
http://www.on.ec.gc.ca/water/greatlakes/raps/intro.html
Environment Canada. 2000. Information Technology (IT) and Telecommunication (Telecom) Waste in Canada.
http://www.ec.gc.ca/nopp/sustainable/itwaste/ITWasteE.pdf
Environment Canada. 2001a (Updated: 2001). Persistent Toxic Substances: Toward Virtual Elimination.
http://www.on.ec.gc.ca/pollution/fpd/fsheets/4005-e.html
Environment Canada. 2001b. National Pollutant Release Inventory, National Overview
Environment Canada. 2002a. Priority Substances Assessment Program, Updated: August 2002 .
http://www.ec.gc.ca/substances/ese/eng/psap/PSL1_chlorinated_paraffins.cfm
Environment Canada. 2002b (Updated: May 2002). National Pollutant Release Inventory. 1999.
http://www.ec.gc.ca/pdb/npri/npri_home_e.cfm and http://www.ec.gc.ca/pdb/npri/NPRI_2000changes_e.cfm
Environment Canada 2002c. National Pollutant Release Inventory data base 2000
http://www.ec.gc.ca/pdb/npri/npri_dat_rep_e.cfm#databases.
Environment Canada, 2002d, Proposed Risk Management Strategy for Nonylphenol and Its Ethoxylates Under
CEPA 1999, (May 2002).
Government of Canada 2002 (Updated). Canada's National Programme of Action for the Protection of the
Marine Environment from Land-Based Activities (NPA).
http://www.ec.gc.ca/marine/npa-pan/issues/pops/faq_e.htm
Environment Canada 2002e. Priority Substances List (PSL1): Synopsis of Assessment Report - Polycyclic
Aromatic Hydrocarbons, Environment Canada. http://www.ec.gc.ca/substances/ese/eng/psap/PSL1_PAHs.cfm
53
Environment Canada 2002f. Priority Substances List (PSL1): Synopsis of Assessment Report - Polychlorinated
Dibenzodioxins and Polychlorinated Dibenzofurans, Environment Canada.
http://www.ec.gc.ca/substances/ese/eng/psap/PSL1_dioxins.cfm
Environment Canada 2002g. Priority Substances List (PSL1): Synopsis of Assessment Report - Chlorinated
Paraffins, Environment Canada. http://www.ec.gc.ca/substances/ese/eng/psap/PSL1_chlorinated_paraffins.cfm
Health Canada 1998. The Health and Environment Handbook for Health Professionals.http://www.hc-
sc.gc.ca/ehp/ehd/catalogue/bch_pubs/98ehd211/98ehd211.htm
Health Canada 2001. Priority Substances List Assessment Report, Nonylphenol and its Ethoxylates,
Environment Canada, Health Canada. http://www.ec.gc.ca/substances/ese/eng/psap/final/reports/NPEs.pdf
LaMP. 2000. Lake Michigan Lakewide Management Plan (LaMP 2000). http://www.delta-
institute.org/publications/PesticidesFS.pdf
Maguire, R.J. 1991, Water Pollut. Res. J. Can, 26: 43.
NCFAP 2000. National Center for Food and Agricultural Policy http://ncfap.org/pesticidedb.htm
NICNAS 2002. National Industrial Chemicals Notification and Assessment Scheme, Alert No. 1.
Perfluorooctanyl sulfonate (PFOS). http://www.nicnas.gov.au/
NPRI Query 2002. Environment Canada. http://www.npri-inrp.com/queryform.cfm).
OECD 2002a. 34th Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides
and Biotechnology (5-8 November 2002). http://www.oecd.org/EN/document/0,,EN-document-0-nodirectorate-
no-4-36823-0,00.html
OECD 2002b. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) And Its Salt, Report tabled by the joint
meeting of the Chemicals Committee and The Working Party on Chemicals, Pesticides and Biotechnology
(ENV/JM/RD(2002)17/FINAL), 21-Nov-2002, 362 pages.
http://www.oecd.org/pdf/M00036000/M00036809.pdf
PANNA 2002. Pesticide Database, Pesticide Action Network North America, PANNA.
http://www.pesticideinfo.org/
Pelletier, E. 1995. Environmental Organomematillic Chemistry of Mercury, Tin, and Lead: Present Status and
Prespectives. In Metal Speciation and Bioavailability in Aquatic Systems, Edited by Tessier, A. and Turner
D.R., John Wiely and Sons, Toronto.
Pest Management Regulatory Agency. 1996. Registration Status of Products Containing Cyanazine. Health
Canada.http://www.hc-sc.gc.ca/pmra-arla/english/pdf/reg/reg_r9601-e.pdf
UNEP 2000. Global Programme of Action Clearing-House Mechanism, United Nations Environment
Programme. http://pops.gpa.unep.org/13chlo.htm
UNEP 2001. Stockholm Convention Persistent Organic Pollutants (POPs), United Nations Environment
Programme http://www.chem.unep.ch/sc/
U.S. EPA 2000a. Lead - How Lead Affects the Way We Live & Breathe, United States Environmental
Protection Agency. http://www.epa.gov/tri/
U.S. EPA 2000b. Superfund: 20 Years of Protecting Human Health and the Environment, United States
Environmental Protection Agency http://www.epa.gov/superfund/action/20years/index.htm
U.S. EPA 2000c. www.epa.gov/ncea/pdfs/dioxin/part3/chapter1-6.pdf
U.S. EPA 2001. Binational Toxics Strategy Mercury Progress Report, October 18, 2001.
http://www.epa.gov/region5/air/mercury/progress.html#chlorine%20production
U.S. EPA 2002a. 2000 Toxics Release Inventory (TRI) Public Data Release Report, United States
Environmental Protection Agency.
54
U.S. EPA 2002b. Toxics Release Inventory (TRI) Program, United States Environmental Protection Agency.
http://www.epa.gov/tri/
U.S. EPA 2002c. U.S. EPA Mercury, United States Environmental Protection Agency.
http://www.epa.gov/mercury/index.html
U.S. EPA. 2002d. Amendment to the Premanufacture Notification Exemptions: Revisions Of Exemptions For
Polymers: 40 CFR 723. http://www.epa.gov/EPA-GENERAL/2002/May/Day-13/g7670.htm
U.S. EPA 2002e. Areas of Concern (AoCs), Great Lakes National Program Office, Chicago.
http://www.epa.gov/glnpo/aoc/index.html
U.S. EPA Workshop 2001. Record of the US EPA Great Lakes National Program workshop on emerging
contaminants for fish contaminant monitoring, March 6 2001, Chicago, IL.
http://www.epa.gov/glnpo/monitoring/prog_projects/new_emergcontaminants.html
U.S. EPA 2002e. U.S. EPA Superfund NPL (National Priorities List) Site Information.
http://www.epa.gov/superfund/sites/npl/info.htm
Voldner E. C. and L. Smith 1986. Production, usage and atmospheric emissions of 14 priority toxic chemicals,
In: Procedings of a Workshop on Great Lakes Atmospheric Deposition, International Joint Commission,
Scarborough, Ontario, Oct. 29-31, Appendix 2.
WHO 1996. Environmental Health Criteria 181: Chlorinated Paraffins, International Programme on Chemical
Safety. http://www.inchem.org/documents/ehc/ehc/ehc181.htm#SubSectionNumber:3.2.1
WWF (World Wildlife Federation) 2000. Toxic Hotspots: Polluting Planet Earth.
http://www.panda.org/toxics/pubs.cfm
55
3. ENVIRONMENTAL LEVELS, TOXICOLOGY AND ECOTOXICOLOGY
PATTERNS
The Prevailing Situation in Mexico
Although, at this time, there is not a continuous monitoring program for the substances of concern in this report,
there are a number of reports from academic research projects, primarily related DDT and its metabolites. As
described in Chapter II, DDT was used as a measure against malaria vectors. Its use has decreased from 1999 to
the present, under a program based in an ecosystemic approach. For this reason, human biological results
(Section 3.3) are divided in those sampled before 1999 and those after 1999, when there no longer were direct
applications of DDT to the environment. Environmental data (Section 3.1) for DDT are presented in two tables,
according with the environmental compartment.
The first three sections of this chapter deal with a) the levels and trends of PTSs in the environment, b) the
ecotoxicology of PTSs and their impacts on the environment and c) the toxicology of PTSs and their impacts on
human health. The last section discusses data gaps.
3.1 Environmental Levels and Trends
The amount of data available on levels and trends in the environment is uneven. For most of North America,
there is little information. The one exception is the Great Lakes basin where PTSs have been extensively studied
for over two decades with more and more chemicals being added to the studies in recent years. The results from
these studies have provided valuable guidance to studies in other parts of North America and have influenced
national policies and control measures. The following brief history of the Great Lakes studies provides a context
for the levels and trends sub-sections that follow.
The Great Lakes Sub-region
In the 1970s it became evident that human population growth and industrial development in the sub-region were
leading to extensive environmental degradation in the basin (IADN, 1998a). A number of bilateral programs
were put in place to study the problem and to provide guidance to the two countries in seeking common
solutions to the deteriorating situation. The results of these early environmental studies were wide-ranging
(Environment Canada 1991):
· As early as 1963 studies of Herring Gulls in Lake Michigan revealed poor reproductive success and high
levels of DDT/DDE.
· In 1968 high concentrations of mercury were found in sediments in Lakes Ontario and Huron. At that time,
industries using the chlor-alkali process were discharging mercury directly into rivers and lakes.
· In 1970 mercury was found in fish from Lake Huron, Lake St. Clair, western Lake Erie, eastern Lake
Ontario and the St. Lawrence River, resulting in the closure of some commercial fisheries on the lakes.
· In 1971 a wildlife biologist reported on a colony of common terns in Hamilton Harbour. Eggs were
observed containing dead embryos and a young chick was observed with a crossed-bill deformity. These
problems were later linked to the presence of PCBs, DDT/DDE and hexachlorobenzene in the eggs.
· In 1971, as a result of finding PCB residues in fish, the Michigan Public Health Department issued the first
advisory in the Great Lakes limiting the consumption of lake trout and salmon from Lake Michigan.
· In 1974, mirex was discovered in fish in the Bay of Quinte. The only known sources were a manufacturing
plant on the Niagara River and another on Lake Ontario.
· In 1976 Ontario introduced a Guide to Eating Ontario Sport Fish (OMOE 2001).
· In 1978 the Love Canal near Niagara Falls, New York, was declared a health emergency and 238
households were evacuated. A mixture of industrial solvents, pesticides and process sludge long abandoned
in an unused disposal canal had seeped its way into residents' backyards. One thousand and thirty
households were evacuated.
56
· During the late 1970s, the word dioxin entered the lexicon as the name of the most toxic chemical found in
the Great Lakes ecosystem.
Throughout this period of examination and assessment, the International Joint Commission (IJC) played a lead
role. The IJC was established by the Boundary Water Treaty of 1909 between the United States and Canada.
The purpose of this treaty was to provide a mechanism for addressing problems shared by the two countries
regarding their common boundary water areas and transboundary rivers. The main focus of the IJC work in the
1960s and early 1970s was the excessive loading of nutrients to the Great Lakes. The nutrients were entering the
lakes through sewage disposal and were associated to a large degree with household detergents.
In 1972, under the IJC, the United States and Canada signed the Great Lakes Water Quality Agreement to
initiate remedial actions to improve the quality of Great Lakes waters, focusing on the issue of excessive
loadings of nutrients. In 1978, the two countries signed a revised Agreement to "restore and maintain the
chemical, physical and biological integrity of the waters of the Great Lakes ecosystems," with an emphasis on
reducing toxic contaminants (IJC 1978). The Agreement was amended again in 1987, expanding many of the
programs and recognizing that an ecosystem approach was required to truly restore and protect the Great Lakes
ecosystems (IJC 1987).
In 1991 the Canadian government published a synopsis of the situation in the Great Lakes entitled Toxic
Chemicals in the Great Lakes and Associated Effects: Synopsis (Government of Canada 1991). This synopsis
provides a benchmark on the condition of the Great Lakes at that time and valuable guidance for further
assessment and remedial action (Environment Canada 1995). The principle findings of the study included the
following:
· The concentrations of toxic chemicals in the open waters of the Great Lakes are well below Canadian and
international drinking water standards.
· The analysis of bottom sediments indicate that peak inputs of synthetic organic chemicals and metals to the
lakes occurred in the 1960s and 1970s followed by declines in the 1980s. Levels of contaminants in Great
Lakes fish and aquatic birds have decreased substantially from the high values reported in 1970s. Since the
early 1980s, contaminant levels in wildlife have remained constant.
· Contaminant-related restrictions on the harvest of many commercial fish species are still in place.
· Fish consumption advisories have been issued in 36 of the 42 highly contaminated areas of concern in the
Great Lakes.
· Multi-media analysis of national and Great Lakes regional data indicate that 80-90 percent of human
exposure to persistent organics comes from food, 5-10 percent from air, and less than one percent from
water.
· There is good circumstantial evidence that contaminants were involved in the mass morality of developing
lake trout embryos in Lake Michigan during the early 1980s.
· There is convincing evidence from laboratory data that toxic chemicals ultimately affect the survival,
growth and reproduction of some fish species in the Great Lakes.
· Eleven wildlife species (Mink, Otter, Double-crested Cormorant, Black-crowned Night-Heron, Bald Eagle,
Herring Gull, Ring-billed Gull, Caspian Tern, Common Tern, Forster's Tern, and Snapping Turtle) in the
Great Lakes basin have experienced reproductive and other problems attributed to chemical contaminants.
All are long-lived fish eating species.
· In the last decade there have been significant recoveries in reproductive success and increases in population
for most of the affected bird species. Serious problems are now confined to a few highly contaminated areas.
· The population increases in two species, the Cormorant and Ring-billed Gull, have been outside the range of
variation normally recorded for vertebrate species. The populations are now 20-40-fold greater than any
recorded historically.
57
· The number of bald eagles on the lower Great Lakes is only a small fraction of what it was in the past. In the
1900s, 50 pairs nested on the shores of Lake Erie. By the 1970s, the few remaining pairs produced no
young.
· Birth defects in young fish-eating birds have been recorded in 10 species in the Great Lakes basin. The
prevalence of crossed bills in cormorant chicks in Green Bay is 22 to 87 times higher than normal.
· When compared to in-land populations, a higher percentage of snapping turtles from the shorelines of Lakes
Ontario and Erie have considerably higher numbers of unhatched eggs and deformed embryos.
Since the 1991 synopsis, the situation in the Great lakes has changed. However, the presence of toxic substances
in the Great Lakes continues to be a significant concern. There have been 362 contaminants identified in the
Great Lakes system; of these 362, approximately one-third have been evaluated for their potential toxic effects
on aquatic life, wildlife, and human health, according to the IJC (Great Lakes Commission 2002).
In 1985, eleven of the most persistent and widespread toxic substances were identified as "critical Great Lakes
pollutants" by the IJC. Because of the persistence and widespread contamination of these Great Lakes pollutants
in the environment, toxic effects in wildlife have been demonstrated and results from epidemiological
investigations suggest that adverse human health effects, (i.e., reproductive, developmental, behavioural,
neurologic, endocrinologic, and immunologic) are associated with exposure to Great Lakes pollutants. (U.S.
EPA 2002a) The critical pollutants are the following:
· Alkylated lead
· Benzo[a]pyrene (a member of a class of substances known as PAHs)
· Dichlorodiphenyl trichloroethane (DDT) and metabolites
· Dieldrin
· Dioxins
· Furans
· Hexachlorobenzene
· Methylmercury
· Mirex
· Polychlorinated biphenyls (PCBs)
· Toxaphene
These chemicals are included in the UNEP list of chemicals, described in Chapter 1. In addition, nine of the
above chemicals are included in the Stockholm Convention list of 12 priority substances. Alkylated lead,
Methylmercury and Benzo[a]pyrene, are not included in the Convention at this time. Hence, the current UNEP
assessment benefits from the intensive work which has been underway in the Great Lakes for over two decades.
In fact, the scope, strategies, research programs and remediation activities undertaken in the Great Lakes Basin
by the two countries could well serve as a model for similar undertaking in other regions. The efforts continue to
produce major achievement, which are not limited to the Great Lakes sub-regions. The Great Lakes program has
had a major influence on national policies and programs to address the PTSs issue. A recent survey of
achievements is included in the 2000 progress report of the Great Lakes Binational Toxics Strategy. The
Strategy provides a binational framework for actions to reduce or eliminate persistent toxic substances,
especially those which bioaccumulate, from the Great Lakes Basin (Great Lakes Binational 2000).
Mountain Sub-regions
In contrast to the extensive research which has focused on the Great Lakes, the mountainous regions of North
America have been neglected. At the same time there is increasing evidence that PTSs have unexpectedly high
levels in the terrestrial and freshwater compartments in mountainous country (Turk et al. 2001, US NPS 2001,
Environment Canada 2002a). The reason for the elevated levels is in part due to the cold condensation, a process
which contributes to the elevated levels in the Arctic (AMAP 1998). Revolatilization is less efficient in cold
climates, such as occur in mountains, and hence, PTSs tend to accumulate there. In addition, precipitation tends
to be enhanced by elevation leading to great web deposition of chemicals. Finally, the temporary (tourist) and
58
permanent populations in mountainous country have increased dramatically in recent decades leading to
increases in the local sources of PTSs. Records show that in the last two decades, the number of visitors to
Rocky Mountain National Park in Colorado has grown 50 percent. At peak season, 20,000 cars a day drive
through Canada's national parks in Banff, Alberta (NGS 2002).
The mountain ranges along the west side of the North America, which stretch from northern British Columbia
southward and cover most of Mexico, constitute a major portion of the UNEP region. Atmospheric, terrestrial
and freshwater pathways and levels and trends in PTSs should be evaluated in a comprehensive and systematic
way to determine the extent of the focusing of contaminant in these zones and the relative importance of long-
range offshore air pathways.
3.1.1 Air and Precipitation
The Great Lakes Sub-region
In the mid-1980s, the early success of the control programs of the late 1970s and early 1980s to lower
contaminant levels in fish seemed to be ending. Monitoring of levels in fish and other biota indicated that a
downward trend of contaminant concentration appeared to be levelling out.
Scientists sought an explanation for the lack of continued reduction in the contaminant levels in biota.
Examinations of lake water and tributaries had been unable to explain the sources of the continuing
contamination. One such explanation was the possibility that the contaminants were coming via the atmosphere
(IADN 1998a). Early studies assessed by the IJC under the Great Lakes Water Quality Agreement (GLWQA)
had previously mentioned the role that the atmosphere might play in maintaining high lake concentrations
(IADN 1998a).
The atmospheric deposition of persistent toxic chemicals to the Great Lakes was first observed in rainfall in the
1970s (Sanderson and Frank 1977; Murphy and Rzeszutko 1977; Strachan and Huneault 1979). The initial focus
was on PCBs, and the observed concentrations, coupled with annual rainfall deposition, indicated inputs of
several tonnes per lake. Other contaminants were found at much reduced levels although all were near the
detection limits at that time (IADN 1998a).
At the start of the 1990s, the Integrated Atmospheric Deposition Network (IADN) was established by the
governments of Canada and the United States. By 1992 the program was in full operation. IADN was
established to determine the magnitude and trends of atmospheric loadings of a range of toxic contaminants to
the Great Lakes (IADN 1998b). Ten to fifteen institutes have been involved in the IADN project over the years.
The institutes are located at universities and government laboratories. The individual participants have changed
from time to time.
By maintaining one master station plus satellite stations on each of the Great Lakes (Figure 4), IADN has been
able to monitor the basin-wide atmospheric concentrations of selected pollutants in precipitation, in particles and
as gases.
By themselves, these concentrations are not sufficient to determine the loadings to the lakes. They only indicate
whether a chemical occurs, its quantity, and its form. What is needed is the net atmospheric flow (kg/yr), into
and out of the lakes, based on the sum of three processes: wet deposition (precipitation), dry deposition, and net
gas exchange, integrated over the whole lake.
IADN incorporates three atmospheric fluxes into its loadings estimates: wet deposition, dry particle deposition,
and net gas exchange which combines the processes of gas absorption (air to water) and volatilization (water to
air) (IADN, 1998b).
59
Figure 5: IADN Master and Satellite Sampling Stations
One starts by calculating the rate of vertical transfer of material per square centimetre the flux. Wet deposition
flux (g/cm2 sec) is calculated directly by multiplying the volume of precipitation per square centimetre per unit
time by the concentration. This is simply the precipitation rate (mm per hour, for example) multiplied by the
concentration. In the case of dry deposition and net gas exchange, the measured concentrations are multiplied by
meteorological variables and various parameters (Hoff, 1996). Multiplying the flux by the area of the lake gives
the flow. Wet and dry depositions deliver materials to the lakes. Only gas exchange, volatilization, can remove
chemicals from the surface.
The calculations are rather complex and subject to some uncertainty. However, when the calculated flow values
are averaged over a season, the procedure can produce reliable and useful estimates of the movement of selected
chemicals into and out of the Great Lakes. By comparing one season with another or one year with another,
trends in loadings can be determined.
Initially, the relative importance of the atmosphere was not known. In 1994, a workshop was held at Windsor,
Ontario, to use the IADN data, along with supplementary information from research efforts on the Great Lakes,
to make estimates (Hoff et al., 1996) of the loadings of toxics chemicals to each of the lakes with data focusing
on the 1992 sampling year (data from 1991-1993 were also consulted). The conclusion drawn in the study was
that gas transfer is the most important, if not dominant, component of the loading for most of the organochlorine
compounds studied (IADN, 1998a).
This work expanded the efforts of the 1988 IJC study by including a wider range of chemicals (14 in all) which
were examined not only for their dry and wet deposition loadings to the lakes, but also for their gas transfer
component.
As might be expected, in the case of chemicals that are still in use, the net result from these processes has been a
flux into the lakes. However, in the case of some banned substances, the air and precipitation moving into the
region has become relatively clean so that the contaminated lake can now act as a source and volatilization
(water to air) is the most dominant process.
60
The suite of chemicals measured by participating agencies has been extensive: 24 organochlorine compounds,
most of the PCB cogeners, 23 PAHs, 8 principle metals (excluding mercury) and 35 trace elements. Not all
substances have been measured routinely or over the full length of the program. In addition, not all chemicals
have been measured at all stations.
A subset of the substances measured at the IADN master stations is used in the loadings calculations. These
substances are the pesticides - and -hexachlorocyclohexane (HCH), dieldrin, p,p'-DDE, p,p'-DDD, p,p'-DDT,
trans-nonachlor, trans- and cischlordane, a-endosulfan, and endosulfan sulfate; hexachlorobenzene (HCB) and
polychlorinated biphenyl (PCB) congeners 18, 44, 52, and 101, as well as a sum of 56 PCB congeners and
coeluting congener groups expressed as "suite-PCB"; the individual polycyclic aromatic hydrocarbons (PAHs)
phenanthrene, pyrene, benzo[k]fluoranthene, benzo[b]fluoranthene, indeno[1,2,3-cd]pyrene and benzo[a]pyrene
as well as a the total of four of these PAHs expressed as sum-PAH; and the trace metals lead, arsenic, selenium,
and cadmium. Mercury is not included (IADN, 1998b).
In 1998, the results of seven years of measurements were published (IADN, 1998b). The findings relate to
fluxes of individual chemical groups, as well as spatial and temporal trends across the entire basin. Some key
findings are described below.
Levels
Downward fluxes for pesticides in 1997 and 1998 ranged from 0.01 ng/m2/day to 40 ng/m2/day, with in- use
pesticides such as -HCH accounting for the highest fluxes. Volatilization fluxes for those pesticides banned
from use were almost 10 times greater than those for currently used pesticides, reaching 37 ng/m2/day at their
highest. PCB and HCB downward fluxes ranged from 0.02 ng/m2/day to 11 ng/m2/day across the basin.
Volatilization fluxes for these banned commercial chemicals were on the same order as those for banned
pesticides. Suite-PCB volatilization fluxes increased from west to east across the basin. Downward fluxes for
PAHs ranged from 0.3 ng/m2/day to 530 ng/m2/day with volatilization fluxes ranging from 0.00001 to 240
ng/m2/day. Where water concentration data are available, volatilization of PAHs was almost always less than net
inputs. Fluxes for metals ranged from 13 to 840 ng/m2/day for dry deposition and from 130 to 5400 ng/m2/day
for wet deposition. Since the metals analyzed by IADN are non-volatile, they are not measured in the gas phase.
The PAHs and metals measured by IADN are currently emitted through anthropogenic means into the
atmosphere and thus have downward (air to water) fluxes much greater than those of the pesticides and PCBs
that have been banned from use (IADN, 1998b).
Atrazine has been measured in rivers, the atmosphere, sediments and lake water, as part of the Lake Michigan
Mass Balance Study, reported in the Lake Michigan Lakewide Management Plan (LMLMP 2002). According
to this report, atrazine in water is decaying only at an estimated rate of 0.8% per year. This translates to a half-
life of approximately 87 years. Further, it was estimated that if loadings of atrazine were to continue into the
future at a rate equivalent to that of 1993, Lake Michigan would reach steady-state concentrations in 300 years.
These findings suggest that atrazine is persistent in fresh water resources.
The Urban Effect - Chicago
In the Great Lakes region, all of the air and precipitation flows and fluxes are based on IADN master station
data. These stations are remote sites, one on each lake, which measure what are considered to be Great Lakes
background contaminant levels. However, spatial differences exist across each lake for many of the compounds
monitored, particularly near urban areas, where atmospheric deposition from cities can be much greater than that
at remote sites. In an attempt to assess the impact of urban areas on lake-wide loadings, depositional data from
IADN's Chicago site were extrapolated onto Lake Michigan loadings. The impact of Chicago pollution on a
small sub-area of Lake Michigan was then compared to loadings calculated at the remote master station.
Results demonstrate that urban inputs have a minor lake-wide effect for most pesticides. There does, however,
seem to be a large effect on cis- and trans-chlordane, drastically changing lake-wide volatilization and markedly
increasing total mass loadings. Urban inputs also have a strong effect on the net gas exchange of PCBs. PAHs,
currently emitted urban pollutants, and show consistently large urban effects in all deposition categories (IADN
1998b).
61
Trends
Current (1997-1998) fluxes (ng/m2/day) were compared across time and space to better understand loadings
trends in the Great Lakes. Pesticide wet deposition fluxes seem to be generally decreasing over time except for
-HCH at Lakes Huron and Ontario. Since -HCH is still in use, this trend is expected. Volatilization of dieldrin
from Lake Ontario is the largest pesticide flux observed. The magnitude of PCB wet deposition fluxes is similar
for Lakes Superior, Erie, and Michigan. Lake Erie, however, seems unique in that all PCBs measured there
reached peak fluxes around 1994 and 1995 and then decreased in the following three years. Gas exchange of
PCBs has been, for the most part, in the direction of net volatilization consistently over time with only Lake
Michigan showing signs of nearing air-water equilibrium. Wet and dry deposition of PAHs shows no real
temporal trend, but spatial analysis indicates that fluxes have increased from west to east across the basin. Gas
exchange fluxes for Lakes Superior and Erie for all PAHs show net absorption over time. Metal fluxes for Lakes
Huron and Ontario are similar over the years with dry deposition showing no real trend and wet deposition
decreasing from 1992-1996 for Cd and Pb, then increasing in 1997 and 1998 (IADN, 1998b).
In an attempt to explore a more tangible means of examining the loadings results, estimates were calculated on a
Great Lakes basin-wide basis by summing the total deposition flow (kg/yr) of each substance over all five lakes
for each year. The results are given in Table 1.
Table 1: Annual flow of substances into or out of the five Great Lakes over a seven year period. (IADN 1998b)
These sums give a good approximation of the larger, regional atmospheric deposition to the Great Lakes. Total
deposition for -HCH showed a decreasing trend, going from 950 kg/yr in 1992 to 210 kg/yr in 1998. Dieldrin
and p,p'-DDE, two organochlorine pesticides banned from use, had negative total deposition across time,
indicating that the lakes are acting as sources of these chemicals to the atmosphere. Sum-PCB total deposition
across the basin also showed net volatilization for all years. Even so, PCB flows out of the Great Lakes have
decreased dramatically over time, with the largest drop occurring between 1994 and 1995 when total PCB flows
went from 3100 kg/yr to 940 kg/yr. PAHs and metals had the largest regional deposition. PAH flows have, for
the most part, remained stable across time. While total loads of metals to the Great Lakes basin have decreased
over time, the region was still receiving 78000 kg of lead in 1998 (IADN, 1998b).
62
With seven years of data, some general trends have emerged over time. Loadings of banned pesticides and
commercial chemicals have decreased over the years, with the lakes themselves actually becoming the main
source of these substances to the atmosphere. These trends also suggest that most of the restricted pesticides and
PCBs measured by IADN are approaching air-water equilibrium. For those chemicals that are currently emitted,
such as PAHs, and some pesticides, their loads have remained relatively constant through time.
National Air and Precipitation Programs
Although there are a number of national air and precipitation programs in the region, none are designed to
provide long-term, scheduled measurement of PTSs of interest. From time to time levels and trends are
examined in one sub region or another for selected chemical over a specific period. There are no ongoing
monitoring programs outside of the Great Lakes.
3.1.2 Freshwater Compartment
Water chemistry and water-related biological communities are treated together in this section. The assessment of
levels and trends of contaminants in freshwater is severely hampered by a lack of data. This situation may be
surprising given that the biological community in fresh waters has received so much attention over the last few
decades. There are at least two reasons for the absence of authoritative water chemistry data bases; low
concentrations and lack of analytical methodologies.
The concentrations of PTSs in water are extremely low, often near the detection threshold. It can occur that the
concentration of organic pollutants in some fish are high enough to cause serious health risks if eaten frequently
while the concentration of the same pollutants in the water itself is so small that the contaminants from 1000
litres of water must be concentrated to get a sample large enough the measure with certainty. The risk of sample
contamination is ever-present. Mercury is particularly difficult to measure properly in waters because of its very
low concentrations.
The difficulty with producing reliable data which are accepted by the scientific community as a whole is
complicated by the non-uniformity of analytical methodologies in use by laboratories. Not all laboratories use
the same methodologies. Hence, the inter-comparison and confirmation of results from several laboratories that
have analyzed the same sample can be unsatisfactory, and hence, the determination by a team of laboratories of
spatial and temporal trends in water chemistry can be unreliable.
In the North American Arctic (part of UNEP Region I), significant declines of PCBs and DDT have been
observed in seabirds (Braune et al. 2001) but the results are mixed in the case of marine mammals. Significant
declines of DDT have been found in ringed seals since the early 1970s (Addison and Smith 1998) but not for
PCBs in blubber of beluga from southeast Baffin Island over the period 1982 to 1996 (Stern and Addison 1999).
Compared to the Arctic, there are very limited time-trend data for POPs in North American fish outside of the
Great Lakes (Muir et al. 2001).
In the case of fish eating birds, a set of Great Lakes studies is reported here (Environment Canada 2000a, 2000b,
2000c and 2001). Birds are particularly useful because they are one step above fish in the food web and hence,
biomagnification tends to result in bird contaminant concentrations which are fairly high and hence, analytical
data which are fairly reliable. In addition, because of the mobility of birds, the study result can be representative
of a fairly large area, depending on the foraging pattern of the species. This feature may be desirable in some
cases. Their mobility can introduce interpretation difficulties, however. Because of migration, contaminant
levels in fish-eating birds may not be due to the Great Lakes alone, but may reflect conditions outside the Great
Lakes basin.
3.1.2.1 The Great Lakes Basin
The Great Lakes maintain a commercial fishing industry as well as sports fishing activities. Subsistence fishing
and sports fishing also occur in hundred on smaller lakes in the basin. In spite of this fishing activity, there seem
to be no information on the number of people in the Great Lakes basin who consume fish from the lakes. This is
an odd situation given the great concern for the quality of the fish and their potential impacts on humans.
63
3.1.2.1.1 FISH
The ultimate purpose in determining the levels of toxic chemicals in fish is to obtain an indication of the
potential risks posed to humans. Since fish flesh is the principal item consumed, research on concentrations of
chemicals have focussed primarily on flesh and muscle tissue. From the perspective of the health of the fish,
these parts of the fish anatomy may be the least sensitive to toxic flows and, hence, the least impacted by toxins.
The fish brain, for example, is far more sensitive to toxins, but this part of the anatomy receives little attention in
terms of levels of contaminants and impacts on the fish.
The levels of many PTSs in fish are decreasing, but still are a concern. The 2001 State of the Great Lakes
Report (Great Lakes Binational 2001) states that lake trout in the Great Lakes, except for Lake Superior, were
eliminated by 4 stressors, over-fishing, habitat loss from development and agriculture, non-native invasive
species such as the sea lamprey and contamination of fish by dioxin-like chemicals peaking in the 1960s.
Contamination levels in young fish from 1945 to 1975 were high enough to cause 100% mortality. The data
show that lake trout abundance has increased since the early 1980s due to reduced Dioxin-like compound levels
and sea lamprey control. Natural reproduction now occurs in Lake Superior and stocking has been discontinued,
however, natural reproduction in the other Great Lakes is very low or non-existent and lake trout populations are
solely maintained by stocking.
Concentrations of PCBs, DDT and other persistent organochlorine pesticides remain high in many aquatic food
webs in Canada as a result of emissions from old sources and from long-range transport and deposition (Muir et
al. 2001). However, in the Great Lakes region, levels of PCBs and DDT have declined significantly in top
predators. For example, continued decline in PCBs in herring gull eggs reflects lower emissions following
controls on open uses. Declines in Great Lakes lake trout and walleye have not been as dramatic especially since
the mid-1980s reflecting continued emissions from urban areas and recycling of contaminants within the lakes
(Pierce et al. 1998).
In the Great lakes, considerable emphasis has been placed on the analysis of polychlorinated dibenzo-p-dioxins
(PCDDs) and polychlorinated dibenzofurans (PCDFs). PCBs have dioxin-like toxicity. The 12 most toxic PCBs
congeners are similar in structure and orientation to the 2,3,7,8-TCDD and therefore are referred to as Dioxin-
like PCBs (DLPCBs). Recently, T. M. Kolic et al. (Kolic et al. 2000) reported on a an investigation into
patterns and relative abundances of these two groups of PTSs based on TEQs (Toxic Equivalent Quantity)
between PCDD/Fs and DLPCBs (dioxin-like polychlorinated biphenyls) in fish found in the Ontario Great
Lakes region. TEQ values are the sum of the TEF (Toxic Equivalent Factor), the relative toxicity of the specific
congener normalized to 2,3,7,8-TCDD, multiplied by the concentration. Dioxin-like biphenyls (DLPCBs) were
found to be present in all samples analyzed. This was not observed for PCDD/Fs. In the northern most Great
Lakes, Superior and Huron, the TEQ values for DLPCBs and PCDD/F are relatively low in comparison to the
Lake Ontario fish samples. In certain heavily industrialized areas such as the case in the Niagara River and
Hamilton Harbour locations, the TEQ ratio of DLPCB to PCDD/F is about 4 in the Niagara River and 9 in the
Hamilton area. The average ratio for all lakes is 6.5 (when the large value of 86 from the Welland River is
omitted). Most of the lakes have similar average values (Superior 4.4, Niagara River 2.9, Lake Ontario 5.3
(Welland data removed)), except for Lake Huron with an average ratio of 11.4 and the Northern areas
(Mattagami and Cochrane). When comparing the PCDD/F TEQ values at locations with the same fish species
against those determined in an earlier study, there is a general decrease in PCDD/F levels in the Ontario region
lakes. With respect to DLPCBs, there is no previous record to compare or determine relative trends. Very little
data for DLPCBs have been published to date. Determination of DLPCBs in other matrices such as
soil/sediment, vegetation, and water would greatly aid in the identification of point and long range
transboundary sources contributing to the different congener pattern distributions that are observed in the
different fish species.
Despite the declining levels, the interim guideline for PCB of 0.32 pg TEQ/g ww, designed to protect Canadian
wildlife that consume fish and shellfish, is routinely exceeded by both predator and forage fish in many areas.
Also, PCB levels in surface waters in many small rivers draining urban areas regularly exceed the 1 ng/L EQG.
In the case of methyl Hg, the interim guideline of Hg of 22 ng/g ww for protection of fish consuming wildlife is
exceeded in almost all fish measured to date in Canada.
64
In Ontario, fish consumption advisories in lakes are provided for many parts of the Great Lakes as well as for
hundreds of inland lakes within and outside the Great Lakes Basin. In 2001, the number of advisories for each
chemical were: A) Great Lakes (714 advisories) - 34% mercury, 42% PCBs, 1.5% dioxins, 12.5%
mirex/photomirex, 10% toxaphene; and B) inland lakes (7499 advisories) - 99% mercury, 1% PCBs (OMOE
2001).
Levels of mercury, unlike the PCBs and DDT, have increased in the past 20 years in fish-eating birds and
mammals. A striking example is the twofold increase from 1975 to 1995 observed in mercury in thick-billed
murre eggs in the Canadian High Arctic (Braune et al. 2001). Another, independent line of evidence that
mercury inputs to remote locations have been increasing comes from profiles in sediment cores. These also infer
that inputs have increased greatly relative to pre-industrial times (Muir et al. 2001).
3.1.2.1.2 FISH-EATING BIRDS
Fish-eating birds are the top predators in the aquatic food chain and can be used to assess chemical
contamination in freshwater and marine environments. In Europe, seabird eggs have been shown to be a very
useful monitoring matrix. The European work on Herring gulls (Laurus argentatus) is of particular interest
because of the great deal of comparable Herring gull work that has been done in the Great Lakes basin (see
below). Herring gull eggs have been collected from the breeding colony on the islands of the North Sea and
Baltic Sera. The islands are located in the estuaries of the river Weser (Island Mellum), River Elbe (Island
Trischen) and River Oder (Island Ruegen, sanctuary Heuwiese). (UNEP RBA of PTS for Region III, Europe. In
preparation)
Herring Gulls
Research and monitoring in the Great Lakes have focused on heavy metals such as mercury, organochlorine
pesticides such as dichlorodiphenyltrichloroethane (DDT), dieldrin and mirex, and other chlorinated organics
such as polychlorinated biphenyls (PCBs), hexachlorobenzene (HCB), dioxins and furans. All of these
contaminants have been detected in Herring Gull eggs and are routinely measured (Environment Canada 2000a).
Today, the Herring Gull continues to be recognized as one of the major indicator species for environmental
contamination in the Great Lakes. The program is one of the longest running wildlife monitoring programs for
contaminants in the world.
Twenty-five years of monitoring contaminant levels in the eggs of Great Lakes Herring Gulls has shown that
concentrations were highest in the early and mid-1970s and that levels from all sites have decreased greatly
since that time. However, it is almost certain that even higher levels of organochlorine contamination occurred
in Herring Gull eggs in the 1960s, prior to the start of this monitoring program. Figure 6 illustrates the trends in
concentrations of PCBs, DDE and dieldrin at all monitoring site over the study period.
In the 1970s, the highest PCB and DDE levels were found in Herring Gull eggs from Lake Ontario, Lake
Michigan and the Detroit River. Lake Michigan Herring Gull eggs were also the most heavily contaminated
with dieldrin. These higher levels of contamination were reflective of the intense agricultural practices
especially in fruit growing areas in Lake Michigan and Lake Ontario, large urban populations, and large
industrialized complexes present around these areas.
Herring Gull eggs from sites in Lake Superior and Lake Erie were generally the least contaminated with PCBs,
DDE and dieldrin compared to the other lakes. The lower contaminant levels in gull eggs from Lake Superior
were probably due to the lower levels of development, industry and human population along its shores, in
comparison with the lower Great Lakes. However, contaminant levels in Lake Superior eggs have not decreased
as fast as levels found in eggs from other regions on the Great Lakes. This is mainly due to two factors. First, the
amount of particulate matter in Lake Superior is very low. Since one of the ways organochlorine compounds are
removed from the water column is through sedimentation of particulate matter, the rate of removal of these
compounds from Lake Superior is slow. Second, unlike the lower Great Lakes, the major pathway for
contamination of Lake Superior has always been the atmosphere. Atmospheric sources are difficult to control
and are global in nature.
The generally rapid decline of most contaminant levels in Herring Gull eggs in the mid and late 1970s was
mainly due to regulations that were implemented in the late 1960s and early 1970s, restricting the use and
65
production of these persistent toxic chemicals. In stark contrast to the declines observed in other organochlorine
contaminants, levels of dieldrin in Herring Gull eggs from all areas on the Great Lakes remained relatively
unchanged.
Figure 6: Trends in Average Concentrations of PCBs, DDE and Dieldrin
in Herring Gull Eggs at Eight Colonies on the Great Lakes
In the 1980s, the decrease in levels of some contaminants in Herring Gull eggs slowed and began to level off.
This stabilization was largely due to different sources of contaminants compared with sources detected in the
1970s. Contaminant problems in the 1970s were due primarily to the production and disposal of chemical
wastes. Most of these point sources have since been controlled. In the 1980s, primary inputs of persistent
contaminants involved sources that were not as easy to control including: leaching from landfill sites via ground
water; disturbance of contaminated lake sediments; and, atmospheric deposition.
Scientists detected 2,3,7,8-TCDD and other dioxins in Great Lakes Herring Gull eggs in 1980. These chemicals
have been routinely measured since 1981. Pre-1980 dioxin levels were measured using eggs collected from
Scotch Bonnet Island (Lake Ontario) and Big Sister Island (Lake Michigan) that had been stored at the Canadian
Wildlife Service Tissue Bank. Levels of 2,3,7,8-TCDD in Herring Gull eggs from these two sites declined
dramatically from the early 1970s. In the early 1980s, two sites had particularly high levels of dioxins in Herring
Gull eggs: Channel-Shelter Island in Saginaw Bay, Lake Huron and Scotch Bonnet Island in Lake Ontario.
Elevated egg levels of 2,3,7,8-TCDD from these two sites were linked to effluents from past production of
2,4,5-trichlorophenol and 2,4,5-trichlorophenoxyacetic acid, and from the disposal of associated wastes at dump
sites. In other areas of the Great Lakes, where levels of 2,3,7,8-TCDD were typically lower, the major source of
this contaminant came from the atmosphere. However, since the mid-1980s, dioxin levels in Herring Gull eggs
from all areas on the Great Lakes have remained fairly constant with highest levels observed in eggs from
Channel-Shelter Island in Saginaw Bay, Lake Huron.
Levels of some contaminants in Herring Gull eggs have remained relatively stable throughout the 1990s, with
no significant changes observed in levels of PCBs and DDE at some Great Lake colonies. A few significant
decreases in levels of dieldrin and heptachlor epoxide have been noted during this period.
66
This relative "steady state" in contaminant levels indicates that these chemicals are still being released and/or
recycled through the Great Lakes ecosystem by individuals, households, municipalities, industry and/or
agriculture. Atmospheric deposition, agricultural land run-off, the slow movement (leaching) of discarded stocks
of pesticides and other chemicals from landfill sites and agricultural soils into the Great Lakes via groundwater,
and the resuspension of contaminated lake/river sediments, continue to be major indirect sources of
contamination. These indirect sources are difficult to control and contribute slow, but continual, contaminant
inputs into the Great Lakes ecosystem. Atmospheric deposition has become an increasingly significant route of
entry of contaminants into the Great Lakes ecosystem, especially in the upper Great Lakes. On Lake Superior,
for example, up to 90 per cent of toxic contaminants entering this lake comes from the atmosphere in the form
of precipitation.
Bald Eagles (Haliaeetus leucocephalus)
By the late 1970s, eagle pairs nesting on Lake Erie's shorelines produced no young (Environment Canada,
2001). Three active pairs were noted on Lake Superior, but they did not reproduce. The situation was equally
dire on Lake Ontario, Lake Michigan, Lake Huron, Lake St. Clair, and along the St. Lawrence River. The birds,
it was discovered, were suffering from exposure to pesticides. DDT was sprayed regularly along wetlands,
shorelines and in agricultural areas, from the late 1940s to the 1970s. The toxic residue left behind interfered
with the eagles' ability to reproduce.
From the early 1980s, coincident with a significant reduction in the use of toxic chemicals around the Great
Lakes, the eagles' natural reproduction rates began to increase. By the late 1980s, however, positive progress
was emerging. DDE levels decreased by more than 50 per cent. Levels of PCBs decreased by as much as 80 per
cent. The resulting reduction in contaminants in Bald Eagle eggs allowed reproductive rates to recover steadily
(Figure 7). Concurrently, private organizations and government agencies developed conservation programs to
protect nesting sites and to re-establish adequate breeding populations.
Figure 7: Concentrations of Chemicals Found in Bald Eagle Eggs on Lake Erie: 1970 to 1994
Today, Great Lakes Bald Eagles are recovering slowly. Across southern Ontario in 2000, 28 eaglets were known
to fledge, or successfully develop to the point of leaving the nest, from 18 of 23 active nests. Current levels of
DDE analyzed from blood samples from nestling Bald Eagles are fairly uniform across the region, whether
taken from birds on Lake Erie, Lake Huron, Lake Nipigon, Lake Superior or Lake of the Woods. The similar
levels may be the consequence of several factors, including the dispersal behaviour of prey species like fish,
gulls and waterfowl, the migratory behaviour of the eagles, and the natural flow of contaminants through the
Great Lakes.
Since 1997, biologists in Ontario examined ailing and dead Bald Eagles, and found elevated levels of mercury
and lead in their bodies. Preliminary results suggest that the accumulated amounts may have been detrimental to
their health (Environment Canada 1998). Since heavy metals do not bind to fatty egg yolks in the way DDT
does, there is little transfer from the female bird to her eggs. Hence, any accumulated burden could have been
the result of a diet of contaminated food. Since birds that consume heavy metals increase their burden as they
age, heavy metals can diminish their life expectancy. In Kejimkujik National Park in Nova Scotia loons,
elevated levels of Hg have been associated with reproductive effects (Evers et al. 1998).
Double-crested Cormorants (Phalacrocorax auritus)
The Double-crested Cormorant population declined dramatically throughout the 1960s and early 1970s
(Environment Canada 2000b). By 1973, the cormorant population in the Great Lakes had declined by 86% and
breeding birds had vanished from Lakes Michigan and Superior. During the late 1960s, scientists discovered
67
that the eggshells of cormorants nesting on the Great Lakes had been thinning since about 1955. By the early
1970s, eggshells were nearly 30% thinner than normal. This had a devastating impact on the cormorant
population. Thin-shelled eggs could not withstand the weight of the incubating bird and would break before
reaching term, killing the embryo. Scientists also discovered that reproductive success (the number of chicks
raised successfully) had declined from a "normal" level of about two chicks per pair to only 0 - 0.2 chicks per
pair. For cormorants to maintain a steady population, the number of young produced each year and eventually
entering the breeding population must match the number of adult deaths. However, this production rate was far
too low to balance adult mortality rates, which probably accounted for most of the dramatic decline in
population levels.
DDE and Eggshell Thinning: At the time, it was suspected that the declining cormorant population was related
to the high levels of toxic contaminants, particularly DDE and PCBs, then present in the Great Lakes. The
symptoms of the decline - widespread reproductive failure associated with moderate to severe thinning of
eggshells, and high frequency of egg breakage - are all characteristic of DDE contamination. Residues of both
DDE and PCBs in cormorant eggs from Lake Huron were found to be the highest known for this species in
Canada from 1968 to 1972. In 1972 researchers discovered that 95% of the eggs in the Lake Huron colonies had
broken or disappeared by the end of the incubation period. Toxic contamination was the most likely cause of the
widespread reproductive failure among cormorants on the Great Lakes during this period. Similar declines and
reproductive failure were noted in other parts of the cormorants range, including Alberta, Minnesota and
Wisconsin.
PCBs and Deformities: In the early 1970s, deformities in several species of water birds began to be reported
throughout the Great Lakes. These included crossed bills, club feet, extra digits, and eye and skeletal
deformities. Bill malformations are one deformity which is clearly developmental (i.e., initiated as the embryo
develops within the egg) rather than the result of accidents or trauma after the bird has hatched.
Therefore, bill malformations are considered reliable indicators of malfunctions in the normal developmental
process and there is strong evidence that PCBs may be responsible. The role of contaminants in the occurrence
of these deformities was investigated by a cooperative Canadian- American study.
In the mid-1970s, cormorant numbers began a dramatic recovery. From 1973 to 1991, their numbers increased
more than 300-fold. During this 18-year period, the average annual rate of increase was approximately 35%,
meaning that the cormorant population was doubling every three years.
At the same time that reproductive success and population size were improving, contaminant levels were
decreasing. The most regularly monitored sites (colonies in Lake Huron) showed DDE and PCB levels in
cormorant eggs decreasing by more than 80% between 1971 and 1989. Similarly significant reductions in one or
both of these compounds have been recorded in several other species of Great Lakes wildlife, including Herring
Gulls, Common and Caspian Terns, Ospreys and Lake Trout. The rapid decline of contaminant levels in the mid
and late 1970s was due mainly to regulations that were implemented in the early 1970s restricting the use and
production of DDT and related pesticides.
Perspective: From 1979 to 1987, the frequency of bill defects in Double crested Cormorant chicks on the
Canadian Great Lakes ranged from 0 to 6.2 (average = 3.9) in every 10,000 chicks. This is a higher frequency
than that found in relatively uncontaminated areas, such as the Canadian prairies where defects are only 0.6 per
10,000; but much lower than that found in extremely contaminated areas, such as Green Bay in Lake Michigan,
where deformities approached 52 per 10,000 chicks. From 1988 to 1992, the frequency of bill defects on the
Canadian Great Lakes ranged from 0 to 3.2 (average = 1.4) per 10,000; clearly the rate of bill deformities is
decreasing in some areas.
Osprey (Pandion haliaetus)
The Osprey is a good indicator species because, unlike some other top predators such as Bald Eagles and River
Otters, it is remarkably tolerant of humans, and will often nest near houses and in areas of relatively heavy
recreational pressure (Environment Canada 2000c).
68
The 1960s and early 1970s were periods of critical population decline for Great Lakes Ospreys. The first
indications that North American Ospreys were experiencing reproductive problems came from New England in
the mid-1960s. Few chicks were being produced and when nests were examined they often contained unhatched
or cracked eggs, or just eggshell fragments. The same phenomena were seen in Ospreys around the Great Lakes
basin.
Chemical analysis of eggs from parts of North America in which Ospreys were reproducing poorly in the 1960s
and 1970s detected a wide range of organochlorine contaminants. These included the organochlorine pesticide
DDT (first used in the mid-1940s), and its breakdown product DDE. Other pesticides such as dieldrin and
chlordane compounds, as well as polychlorinated biphenyls (PCBs) and mercury also appeared in Osprey eggs
and various body tissues such as liver, brain and muscle.
Eggs collected in the early 1970s from 20 Osprey nests in northeast Michigan and northwest Ontario contained
many of the same contaminants, but for most compounds concentrations were usually below the suspected
critical levels. The minimum critical levels of the main contaminants in the eggs of birds of prey (above which
adverse reproductive effects are frequently observed) are approximately four ppm for DDE, one ppm for
dieldrin, 50 ppm for PCBs, and 0.5 ppm for mercury. However, DDE levels in over two-thirds of these eggs
exceeded four ppm the critical level associated with 15 per cent eggshell thinning and an elevated risk of egg
breakage. In other Canadian provinces and territories about three quarters of Osprey eggs analyzed in the 1960s-
70s also contained more than four ppm DDE, suggesting that eggshell thinning, and the poor reproduction that
resulted, were not confined to the Great Lakes basin.
A single pesticide, DDT, appears to have been largely responsible for the dramatic population declines in
Ospreys and many other bird species. The mid-1970s represents a turning point for Ospreys in the Great Lakes
basin, and in other parts of North America. Since 1972, DDT use had been severely restricted, and Ospreys have
been able to increase their reproductive output to above the break-even point of 0.8 young per pair.
Consequently, Great Lakes Osprey populations have increased dramatically up to the present day. In the early
1990s, surveys in all U.S. and some Canadian parts of the Great Lakes basin recorded at least 750 occupied
Osprey nests. The actual population is even larger since most of northern and central Ontario was not surveyed.
A wide range of persistent toxic chemicals is still found in Osprey eggs and chicks from all parts of the Great
Lakes breeding range. Declining DDE levels since the early 1970s have accompanied increasing eggshell
thickness. Studies in the early 1990s found very few eggs contained more than four ppm DDE, or had eggshells
more than 10 per cent thinner than those prior to the introduction of DDT. Broken eggs are no longer a regular
sight in Great Lakes Osprey nests.
Levels of most organochlorine pesticides in Osprey eggs from the Great Lakes basin have declined over the past
20 years, although a few eggs today still contain contaminant residues at similar levels to those seen in the early
1970s. In contrast to other chemicals, PCB concentrations in eggs have declined only slightly. Other
compounds, such as polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs),
may have much greater toxicity even though they occur at much smaller concentrations. At this time the sub-
lethal effects of many contaminants are poorly understood.
Recent studies indicate that most contaminants occur at below suspected critical levels in Osprey eggs, but eggs
and chicks in Great Lakes nests are usually more highly contaminated than those further inland. Fish and other
fish-eating birds show similar patterns of geographic variation of these contaminants, suggesting that Ospreys
are accumulating toxic chemicals from the local food web.
In 1992, concentrations of the most toxic PCDD (2,3,7,8-TCDD), and PCDF (2,3,7,8-TCDF), were generally
higher in Osprey eggs from Lake Huron than from those further inland. Recently, much higher levels of these
compounds have been found in Osprey eggs from the vicinity of pulp mills in British Columbia, and in eggs of
Herring Gulls and Bald Eagles on the Great Lakes.
Mercury levels have changed relatively little over the past 20 years, and have not exceeded the critical 0.5 ppm
level in any Osprey egg from the Great Lakes basin. Average levels were highest at Ogoki Reservoir, north of
Lake Superior, which had been dammed after World War II.
69
3.1.2.2 Lake Erie Studies
In 1987, under the amended The Great Lakes Water Quality Agreement, Canada and the USA established the
Lakewide Management Plans (LaMPs). The LaMPs are responsible for evaluating impairments of beneficial use
of various natural resources caused by priority pollutants.
In the previous sections, the levels and trends of contaminants in biota have been discussed across the entire
basin. Under the LaMPs an assessment was made of deformities and reproductive impairment of wildlife on one
of the Great Lakes, Lake Erie. Impairments were assessed using epidemiological criteria, species-specific
chemical criteria for tissue concentrations associated with reproductive effects in field and (or) laboratory
studies (Grasman, K.A. et al. 2002).
Bald Eagles
Bald Eagles nesting within eight km of Lake Erie have impaired reproduction. Although Bald Eagle
productivity, the number of fledglings (survival to age of flying) per nest, has increased since 1980 as noted
earlier, the Ohio Lake Erie eagles remain below the recovery goal of 1.0 young fledged per occupied nest.
Increases in the reproductive success of the Lake Erie basin Bald Eagle subpopulation may have been influenced
by several factors that potentially confound linkages between exposure to contaminants in Lake Erie and
biological effects. A large number of uncontaminated nestlings were introduced to the subpopulation through
hacking and fostering projects along the Ontario and Ohio shorelines during the mid-1980s. There appears to
have been a great increase in nesting success within the time period associated with the sexual maturation of
these introduced birds. The recovery of the Bald Eagle throughout North America, including inland areas near
the Great Lakes, has created a pool of young eagles dispersing to find available and unclaimed breeding
territories. The high turnover of adults along the Ohio and Michigan shorelines of Lake Erie suggests the
possibility of colonization of these territories by eagles raised elsewhere. Reproductive success tends to increase
following replacement of an older adult in a shoreline territory (Grasman, K.A. et al. 2002).
Because human interventions and natural immigration have potentially led to the introduction of adult eagles
that were not exposed developmentally (i.e., in ovo) to Lake Erie contaminants, a risk assessment based on
comparison of contaminant concentrations in eagle eggs to known adverse effect concentrations is particularly
insightful. Eagle eggs from the Ohio and Ontario shorelines of Lake Erie consistently exceed PCB and dieldrin
criteria, and often exceed the p,p'- DDE criterion for adverse effects in this species. These Lake Erie data, when
interpreted in the context of studies showing effects of organochlorines on eagle reproduction elsewhere in
North America, strongly suggest that current concentrations of organochlorines in Lake Erie are causing
reproductive impairment of Bald Eagle reproduction (Grasman, K.A. et al. 2002).
3.1.2.3 Eastern North America
A recent Canadian study examined levels of persistent bioaccumulative toxic substances (e.g., PCBs, DDT,
toxaphene and mercury) in top predator fishes and their food webs from lakes stretching over three thousand
kilometres across Canada from northern Alberta to Labrador, and south a thousand kilometres from Labrador to
upstate New York (Muir et al. 2002a).
A total of 34 lakes were studied over a three year period. Twenty-four of the lakes had lake trout as the top
predators and this species was the largest group analysed for contaminants (324 samples). Brook trout, lake
whitefish, walleye, and pike were also analysed from 12 lakes. In 18 of these lakes, scientists examined
biomagnification of POPs and mercury in the food web by collecting forage fish (yellow perch, lake herring,
cisco, lake whitefish, smelt), zooplankton, amphipods (mysis), and benthic invertebrates (Muir et al. 2002a).
3.1.2.3.1 CONCENTRATIONS
This study adds significantly to the data on concentrations within the Great Lakes basin, reported above.
Approximately 860 fish and invertebrate samples were analysed for PCBs, and organochlorine pesticides. The
results are shown in Figure 7. Concentrations of most POPs were low compared to the Great Lakes. Highest
levels of both PCBs and DDT were found in fish from locations nearest to urban areas and municipal industrial
waste treatment centers. PCB concentrations (PCB = sum of 57 congeners) ranged from 1.4 to 1000 ng/g ww
in lake trout and were generally much lower in other fishes. Median concentrations of PCB in (whole) pike and
70
walleye ranged from 5.2 to 38, respectively. Median PCB concentrations in benthic and pelagic invertebrates
(excluding zooplankton) were < 1ng/g ww (Muir et al. 2002a).
Elevated DDT concentrations were found in fish from Wabush Lakes in Labrador presumably due to past use
for biting fly control, and in some isolated lakes in Ontario (Opeongo, Eva and Thunder). P,p'-DDE/DDT
ratios did not vary significantly among lakes which suggests that these were old DDT sources. However the
extent of contamination by DDT was surprising given that these fish were collected 30 years after cessation of
use (Muir et al. 2002a).
71
PCB
DDT
t
)
t
)
w
d 6000
d w 8000
lipi
6000
4000
lipi
g 4000
ng/g 2000
ng/ 2000
(
(
0
onc
0
onc
C
C
1000 k
m
Sampling site (1998-01)
-Endosulfan
Lindane
t
) 20
t
) 12
w
w
i
d 15
i
d
l
i
p
l
i
p 8
g 10
g
g/
4
n
ng/
( 5
(
nc
onc 0
o
C
C 0
Figure 8. Geographic trends of DDT, PCBs, endosulfan and lindane in lake trout from 23 lakes in Canada and northern USA.
Bars represent weight adjusted geometric mean lipid weight concentrations upper 95% confidence interval
72
3.1.2.3.2 SPATIAL TRENDS
Current use pesticides, endosulfan and lindane, were detectable in all lake trout with ranges from <0.1-0.8 ng/g
ww and <0.1-0.6 ng/g ww, respectively. Mean concentrations of lindane and endosulfan in lake trout were
significantly correlated with longitude but not with latitude. Endosulfan was higher in Labrador lakes and
lindane in the west (Figure 8 above). These findings illustrate the wide dispersal of these chemicals from areas
of use to isolated lakes and they agree with known use patterns; endosulfan use is heaviest in eastern Canada
while lindane is used primarily in Saskatchewan (Muir et al. 2002a).
3.1.2.3.3 BIOMAGNIFICATION
Concentrations of major POP groups and most individual components in food web organisms and lake trout
from 14 lakes were found to increase significantly with trophic level, as indicated by nitrogen stable isotope
ratios (15N) in the organisms. Food web magnification factors (FWMFs) for most POPs varied within a
relatively narrow range of about 2-fold among the 14 lakes indicating similar rates of accumulation and
confirming that this parameter is relatively independent of lake size and geographic location. Concentrations of
PCB, as well as HCH, CHL and CBz, in lake trout, as well as FWMF for PCB, were positively
correlated with proportions of the algae Peridineae and negatively related to phytoplankton biomass and
chlorophyll a. The results suggest that the rate of food web biomagnification of many OCs is higher in lakes
with low growth rates due to low light, low temperatures and low nutrients. These are the first observations on
the influence of water chemistry and phytoplankton composition on biomagnification of POPs in North
American lakes and confirm observations that lakes in southern Sweden dominated by cyanobacteria were less
likely to have elevated concentrations of PCBs in both particulate matter and fish (Muir et al. 2002a).
3.1.2.3.4 MERCURY
Mercury was determined in 1002 fish and invertebrate samples, in the same fish that were analysed for POPs as
well as in additional samples. Mercury levels in lake trout muscle ranged from 0.13 to 1.3 ug/g ww and were
significantly different among lakes even after adjusting for size differences. Average mercury concentrations in
lake trout in most lakes exceeded the 0.2 ug/g guideline established for frequent consumers of fish and
concentrations in larger fish (700 mm) in many lakes exceeded the 0.5 ug/g guideline for the commercial sale.
However, average mercury concentrations in insectivorous fish such as lake whitefish and white sucker were
well below this guideline. Higher mercury levels were found in lake trout from lakes impacted by reservoir
creation (e.g. Smallwood reservoir in Labrador), mining effluent (Wabush Lake), and nearby urban sources
(Cayuga, Champlain) (Muir et al. 2002a).
Combining the mercury results from lake trout in NWT and this study shows that there is a "U-shaped"
relationship of mercury concentration as a function of latitude in Canada. At lower latitudes, near urban centres,
mercury concentrations are elevated as a function of anthropogenic impacts localized mercury inputs
(atmospheric and effluents) as well as due to piscivory in lake trout population in these productive lakes. To the
north, away from the urban centres and into a region of relatively pristine lakes and intense commercial, sport,
and domestic fisheries, mercury concentrations decrease. Then, continuing north into even more pristine
environments, mercury concentrations increase again as a function of older, less-intensely fished populations,
many of which are remnant populations inhabiting small glacial lakes (Muir et al. 2002a).
This study has also added significantly to data for mercury in food webs of lake trout lakes combined with
information on water chemistry and phytoplankton composition. Methyl mercury was determined in 131
invertebrate samples so that results consistent with the major form of mercury in fish were available to study
trends in the food web. Predaceous invertebrates (zygopteran, dragonflies, Diporeia and Mysis) were
consistently higher in methyl mercury than the herbivorous organisms sampled (snails, trichopteran, and
chironomids). Length adjusted mercury concentrations in lake trout were negatively related to particulate carbon
and nitrogen and accumulation rates were negatively correlated to particulate P and to proportions of the algae
cryptophycea. These data suggest that mercury concentrations and rates of accumulation in lake trout are lower
in lakes that have higher phytoplankton productivity hence higher particulate carbon and nitrogen. All
regressions of mercury vs. 15N in 17 lake trout lakes were highly significant with FWMFs for mercury of 1.49
to 1.96 indicating the biomagnification of mercury in these lakes. FWMF for mercury were weakly correlated
with phytoplankton composition (% Chryptophycea) and intercepts of the log Hg versus 15N relationship were
73
weakly negatively correlated with particulate carbon. These results suggest that rates of mercury
biomagnification in the food web are relatively unaffected by nutrient status and phytoplankton composition,
unlike the results for POPs (Muir et al. 2002a).
3.1.2.4 Sediments
Changes of chemical deposition in time are expected to be different (1) at different locations (peak
concentrations are expected to occur delayed and dampened at greater distance from the source), and (2) for
different chemicals (the extent of the delay and dampening should be related to physical-chemical properties)
(Wania and Mackay 1996). Unlike data on PTSs in air or plants, dated sediment cores can provide evidence of
the timing and extent of peak concentrations. The determination of historical profiles and concentrations of
pollutants in dated sediment cores has provided valuable information on sources, time trends and current inputs
of PAHs (Heit et al. 1981; Gschwend and Hites 1981), PCBs and chlorinated dioxins/furans (Smith et al. 1993;
Swackhamer and Armstrong 1986; Pearson et al. 1997a, Czuczwa and Hites 1984) and toxaphene (Pearson et al.
1997b).
In 1998 US EPA made a report to Congress on contaminated sediment titled, The Incidence and Severity of
Sediment Contamination in Surface Waters of the United States (US EPA 1998). The report identifies areas in
the continental United States where sediment may be contaminated at levels that may adversely affect aquatic
life and human health. This database includes approximately two million records for more than 21,000
monitoring stations located in nearly 1,363 of the 2,111 watersheds in the continental United States. The
evaluation of the data shows that sediment contamination exists in every region and state of the country and
there are 96 areas (watersheds) of probable concern. More than two-thirds of these watersheds already have an
active fish consumption advisory in place. Water bodies affected include streams, lakes, harbors, near-shore
areas, and oceans. At the Tier 1 level, PCBs, mercury, organochlorine pesticides, and polyaromatic
hydrocarbons are the most frequent chemical indicators of sediment contamination. Approximately 10% of the
sediment underlying U.S. surface waters is sufficiently contaminated with toxic pollutants to pose potential risks
to fish and to humans and wildlife that eat fish. Much of the contaminated sediment in the U.S. was polluted
years ago by such chemicals as DDT, PCB's, and mercury, which have since been banned or restricted.
However, they can persist for many years in the sediment, and continue to be a source of concern for the
environment. Some other chemicals released to surface waters from industrial and municipal discharges and
polluted runoff from urban and agricultural areas continue to accumulate to harmful levels in sediment.
Sources can be inferred from spatial gradients in deposition away from urban/industrial areas or point sources,
from the pattern and correlations of contaminants, and from the date of onset of appearance of the contaminant
in the core (Hites et al. 1977)
Dated lake sediment cores have recently been used to infer present and past inputs, and sources, of PTSs
(organochlorine pesticides, PCBs, chlorinated dioxins/furans and PAHs) as well as heavy metals to sub-Arctic
and Arctic lakes in Canada and Alaska (e.g., Muir et al. 1996; Lockhart et al 1995, 1998; Gubala et al. 1995).
These results are discussed in the UNEP Region I report on the Arctic.
3.1.2.4.1 GREAT LAKES BASIN
Chapter 2, Section 2.4, discussed Areas of Concern in the Great lakes. The Great Lakes program has identified
polluted sediments as the largest major source of contaminants to the Great Lakes food chain, including each of
the 43 Areas of Concern (U.S. EPA 2002c). The work being done at these site concerns remediation. The levels
and trends of contaminants at these locations are monitored to determine the progress being achieved.
3.1.2.4.2 THE DETROIT RIVER
Suspended sediments from the Detroit River were collected in 1999 and 2000 (Marvin et al. 2002.) using
sediment traps at sites ranging from western Lake Erie to southern Lake St. Clair and analyzed to determine the
spatial distributions of contaminants including polychlorinated dibenzo-p-dioxins and dibenzofurans
(PCDDs/PCDFs), dioxin-like PCBs (DLPCBs) and polychlorinated naphthalenes (PCNs). Concentrations of all
three contaminant classes were clearly elevated at sites in the lower reaches of the river in the Trenton Channel.
The potential influence of the Trenton Channel as a source of contamination to western Lake Erie was further
evidenced by PCDD/PCDF homologue profiles, which indicated a contribution from chemical manufacturing in
74
addition to the normal background combustion profile. Toxic equivalents (TEQs) for PCDDs/PCDFs generally
exceeded those for DLPCBs; combined total TEQs in July 2000 for these two compound classes ranged from
2.30 pg/g in southern Lake St. Clair to 306 pg/g at a station just downstream of the outflow of Monguagon
Creek in the Trenton Channel.
3.1.2.4.3 LAKE ONTARIO
Chris Marvin et al. (2002) reported on a 1998 survey conducted to assess spatial and temporal trends in
sediment contamination in Lake Ontario. The survey was also designed to assess changes in environmental
quality since the advent of measures to reduce pollutant discharges and to assist in the tracing of potential
sources and vectors of contaminants. The suite of PTSs analyses included polychlorinated dibenzo-p-dioxins
and dibenzofurans (PCDDs/PCDFs), dioxin-like PCBs (DLPCBs), toxaphene and polychloro-n-alkanes (PCAs).
The analysis showed that the lake-wide average sediment concentration of PCDDs/PCDFs was 91 pg/g TEQs
(2.81 ng/g); highest contamination was observed in depositional areas of the major lake basins. Four sites
exhibited concentrations in excess of 200 pg/g TEQs. Based on analysis of a core sample from the central basin,
accumulation of PCDDs/ PCDFs increased during the 1930s and 1940s; the greatest contamination occurred in
the early 1950s to the late 1960s. Levels declined from the late 1960s to the early 1980s; further declines since
the 1980s are not apparent. Assessment of homolog profiles and ongoing bottom sediment and biomonitoring
have implicated point source discharges within the Niagara River as primary contributors to PCDD/PCDF
contamination in Lake Ontario.
The spatial distribution of toxaphene was similar to PCDDs/PCDFs with sediment concentrations ranged from
0.09 ng/g to 7.7 ng/g with a lake-wide average of 2.6 ng/g. The two-most prevalent individual toxaphene
congeners were B6-923 (Hx-Sed, 0.6 pg/g to 160 pg/g) and B7-1001 (Hp-Sed, 1.2 pg/g to 260 pg/g). Other
prevalent congeners included B7-1450 and B7- 1474+B7-1440. Average relative contributions of hexa, hepta,
octa and nona homolgs were 14 %, 54 %, 30 % and 3 %, respectively. Ratios of individual congeners were also
investigated; Muir et al. (1999) reported that a ratio of B6-923 to B8-1413+B9-1679 <2 to be characteristic of
atmospheric inputs. In general, samples within the deepest areas of the depositional basins exhibited the highest
toxaphene concentrations and B6-923 to B8-1413+B9-1679 ratios >2, while other sites exhibited lower
toxaphene levels and ratios <2. These data suggest that different processes including atmospheric deposition
influenced toxaphene distributions in offshore depositional vs. inshore sediments of Lake Ontario.
The distribution of DLPCBs was similar to PCDDs/PCDFs. DLPCBs included 4 coplanar (BZ# 77, 81, 126,
169) and 8 mono-ortho substituted congeners (BZ# 105, 114, 118, 123, 156, 157, 167, 189). The lake-wide
average DLPCB concentration (9.4 pg/g TEQs) was roughly 10-fold lower than PCDDs/PCDFs. These data are
in contrast to those reported for fish; Lake Ontario TEQ ratios of DLPCBs to PCDDs/PCDFs are roughly 4 on
average5, indicating that DLPCBs are bioaccumulated to a greater extent than PCDDs/PCDFs. Relative
concentrations among coplanar congeners were 77>126>169, however, PCB 126 generally accounted for more
than 75 % of sediment TEQs.
3.1.2.4.4.EASTERN NORTH AMERICA
In 2002, D. Muir et al. published one of the first reports which examined temporal trends and fluxes of PTSs in
sediments in atmospherically impacted lakes in eastern Canada and the north-eastern portion of the United
States (Muir et al. 2002b). The study is extensive and comprehensive. The analyses are not yet completed. Only
a few of the major findings are resented here.
A major focus of the study was the examination of the fluxes and geographical extent of new and emerging
PTSs along a latitudinal and longitudinal gradient. The emerging PTSs included brominated diphenyl ethers
(BDPEs), short-chain chlorinated paraffins (SCCPs), polychlorinated naphthalenes, and pesticides such as
endosulfan and mehoxychlor (a DDT analog). The lakes sampled, shown in Figure 9, stretch from north-east
United States northward to the top of Ellesmere Island, a distance of over 4,500 km. (Muir et al. 2002b).
75
Col ection sites 1999 -2001
Figure 9: Location of Lakes with Datable Sediment Cores for PTSs
The east-west distance is nearly 2,500 km. In all, 52 lakes were sampled for sediment cores and water chemistry
over the period 1998-2001. Not all chemicals were examined in each lake. Clearly, some of the lakes were
outside UNEP Region II, being in UNEP Region I. (Muir et al. 2002b).
In the case of POPs, sediment cores were collected from 25 of the lakes in the study. Large water samples (19L)
were collected from 32 of the lakes for analysis of current use pesticides and 40 lakes were sampled for analysis
of haloacetic acids (HAAs). Water samples were collected on the same lakes as those in which sediment was
collected. The current use organochlorines were endosulfan and lindane. They were measured in 45 lakes (Muir
et al. 2002b).
Sediment core slices, dated to post-1920, were analysed for about 120 individual organochlorine (OC)
compounds. PCBs and DDT-related compounds were the dominant OC contaminants in all cores. Selected cores
were analysed for brominated diphenyl ethers and short chain chlorinated paraffins. (Muir et al. 2002b).
Initial results from this study, some of which are shown in Figure 10, include the following:
76
0.3
Dacthal
20
Metolachlor
15
0.2
10
ng/L 0.1
ng/L 50
0.0
Collection site 1999 -01
Col ection site 1999-01
Dacthal use area
Metolachlor use area
Atrazine and
140
16
120
Flutriafol
100
L 12
desethylatrazine
80
8
60
ng/
4
ng/L
40
20
0
0
atrazine
desethylatrazine
Collection site 1999 -01
Collection site 1999 -01
atrazine use area
flutriafol use area
Figure 10: Dacthal, flutriafol, metolachlor and atrazine/desethylatrazine (ng/L) in large volume water samples - mid-summer - 1999-2001
77
Spatial Trends
· The significant decline from south to north illustrates the importance of urban sources of many of these
compounds e.g. PCBs, chlorobenzenes, and BDE 209.
· Old sources of DDT, such as agricultural lands of southern Ontario and Québec may be important for
explaining latitudinal trends of this OC group and other old pesticides.
· The 34-125-fold difference in fluxes of BDE 209 observed between remote temperate zone and polar zone
lakes is consistent with the much lower mobility of BDE 209 that would be predicted from its physical
properties.
· The sharp latitudinal decline in BDE 209 flux is consistent with its transport mainly on particles.
This study has also conducted the first detailed investigation of current use pesticides, existing and emerging
PTSs in waters of remote lakes in mid-latitude lakes in eastern and central Canada.
· Results for PTSs showed that current use OCs, endosulfan and lindane were present in waters of isolated
lakes in Ontario and New Brunswick and that DDT was still elevated in small forest lakes in New
Brunswick where DDT was applied more than 30 years ago.
· Muir et al. (2002b) have shown that high volume herbicides atrazine, metolachlor and alachlor are reaching
remote lakes and persisting at low ng/L concentrations With the recent observations that concentrations as
low as 100 ng/L affect sexual development in frogs (Hayes et al. 2002), the Muir et al. results suggest that
ecologically important aquatic systems in non-agricultural areas of central Ontario and Québec (i.e. within
100's of km of use areas) could have levels approaching that threshold. The impact of these low level
concentrations, which probably occur annually due to continued atrazine use, needs further investigation.
Identification of New Potentially Persistent Chemicals
· Dacthal appears to have ideal properties for long range transport and deposition, most notably a predicted
long atmospheric half-life. (Figure 9)
· Flutriafol, a fungicide used on cereal crops, was present in lakes northeast of probable use areas.
Information on the quantities of use and the persistence of flutriafol are not available. (Figure 9)
· The presence of perfluorocarboxylates in surface waters of almost all lakes in this study, including
perfluorooactanoic acid (PFOA) was a surprising observation because, as anions, they would not be
expected to be transported long distances. The results suggest that there are atmospheric sources for the
PFAs, and that the pathway must include a volatile precursor. An alternative explanation is that the PFAs
are transported on atmospheric aerosol particles like other anions (nitrate, sulfates).
· Given the apparent high persistence of all PFAs due to the perfluorinated carbon chain these pathways
deserve further study.
3.1.3 Summary of Trends
3.1.3.1 Temporal
Abiotic
Pesticide wet deposition fluxes seem to be generally decreasing over time except for -HCH at Lakes Huron and
Ontario. -HCH is still in use.
The PCB wet deposition fluxes reached peak fluxes around 1994 and 1995 and then decreased in the following
three years. Gas exchange of PCBs has been, for the most part, in the direction of net volatilization.
Wet and dry deposition of PAHs shows no real temporal trend, but spatial analysis indicates that fluxes have
increased from west to east across the basin.
Metal fluxes for Lakes Huron and Ontario are similar over the years with dry deposition showing no real trend
and wet deposition decreasing from 1992-1996 for Cd and Pb, then increasing in 1997 and 1998 (IADN, 1998b).
Annual flow of substances into or out of the five Great Lakes over the period 1992 to 1998, indicated:
78
· Total deposition for -HCH showed a decreasing trend
· Dieldrin and p,p'-DDE had negative total deposition across time, indicating that the lakes are acting as
sources of these chemicals to the atmosphere.
· Sum-PCB total deposition across the basin also showed net volatilization for all years. Even so, PCB flows
out of the Great Lakes have decreased dramatically over time, with the largest drop occurring between 1994
and 1995.
· While total loads of metals to the Great Lakes basin have decreased over time, the region was still receiving
78000 kg of lead in 1998.
· Loadings of banned pesticides and commercial chemicals have decreased over the years, with the lakes
themselves actually becoming the main source of these substances to the atmosphere.
Biotic
In the Great Lakes Sub-region, contaminant levels in fish decreased in the late 1970s and early 1980s due to
control programs. Thereafter, the downward trend of contaminant concentration appeared to level out.
Levels of PCBs and DDT have declined significantly in top predators. For example, continued decline in PCBs
in herring gull eggs reflects lower emissions following controls on open uses. Declines in Great Lakes lake trout
and walleye have not been as dramatic especially since the mid-1980s reflecting continued emissions from
urban areas and recycling of contaminants within the lakes.
Levels of some contaminants in Herring Gull eggs have remained relatively stable throughout the 1990s, with
no significant changes observed in levels of PCBs and DDE at some Great Lake colonies.
Levels of mercury, unlike the PCBs and DDT, have increased in the past 20 years in fish-eating birds and
mammals. However, in Osprey eggs, mercury levels have changed relatively little over the past 20 years and
have not exceeded the critical 0.5 ppm level.
In stark contrast to the declines observed in other organochlorine contaminants, levels of dieldrin in Herring
Gull eggs from all areas on the Great Lakes remained relatively unchanged. Nevertheless, a few significant
decreases in levels of dieldrin and heptachlor epoxide have been noted during this period.
Since the mid-1980s, dioxin levels in Herring Gull eggs from all areas on the Great Lakes have remained fairly
constant with highest levels observed in eggs from Channel-Shelter Island in Saginaw Bay, Lake Huron.
Levels of most organochlorine pesticides in Osprey eggs from the Great Lakes basin have declined over the past
20 years, although a few eggs today still contain contaminant residues at similar levels to those seen in the early
1970s. In contrast to other chemicals, PCB concentrations in eggs have declined only slightly
3.1.3.2 Spatial Trends
Biotic
Concentrations in fish were compared across the central and eastern part of North America, from Alberta to
upper New England. The results were:
· The concentrations of most PTSs were low compared to the Great Lakes. Highest levels of both PCBs and
DDT were found in fish from locations nearest to urban areas and municipal industrial waste treatment
centers
· For current use pesticides, endosulfan and lindane, mean concentrations were detectable in all lake trout and
with were significantly correlated with longitude but not with latitude. Endosulfan was higher in Labrador
lakes and lindane in the west in agreement with known use patterns; endosulfan use is heaviest in eastern
Canada while lindane is used primarily in Saskatchewan.
· At lower latitudes, near urban centres, mercury concentrations in fish are elevated as a function of
anthropogenic impacts.
Abiotic
Concentrations in sediments were compared along a transit from north-eastern lakes (in UNEP region I) lakes
southward down to upper New England. The results were:
79
· Current use OCs, endosulfan and lindane were present in waters of isolated lakes in Ontario and New
Brunswick.
· The high volume herbicides atrazine is reaching remote lakes and persisting at low ng/L concentrations.
· At lower latitudes, near urban centres, mercury concentrations are elevated as a function of anthropogenic
impacts.
· DDT was still elevated in small forest lakes in New Brunswick where DDT was applied more than 30 years
ago.
3.1.4. Marine Compartment
3.1.4.1. Biota
The coastal lagoons of the subtropical Mexican Pacific are among the most productive ecosystems particularly
for shrimp fisheries. However, the increase of human settlements, the agricultural wastes as organochlorine
pesticides and the direct discharges of sewage into the lagoons have given rise to severe pollution jeopardizing
the ecology and biodiversity of these singular areas.
In 2000, Botello et al. (Botello et al. 2000) reported on a study of pesticide pollution in coastal lagoons caused
by the intensive use of agrochemicals and their accumulation in sediments and bioaccumulation in fish and
shrimps employed for human consumption. The study includes two of the most important coastal lagoons in the
littoral fringe of Chiapas State, in the southwest of Mexican Pacific. These are the Chantuto-Panzacola (92°45'-
92° 55' W longitude and 15°09'-15°17' N latitude) with 12 sampling sites and Carretas-Pereira (93°06'- 93°15'
W latitude and 15°23'- 15° 32' N latitude) with 8 sampling sites. Samples from the dry seasons (April 1997 and
February 1998) and the one wet season (July 1997) were compared. The two lagoons are about 50 km apart.
The highest concentrations of organochlorine pesticide occurred during the dry season (February 1998), at 854
ng / g in Carretas-Pereira. During the wet season (July 1997) the highest value was 161 ng / g in Chantuto-
Panzacola. On any sampling occasion there was considerable variation from station to station.
The compounds most often found in the Chantuto-Panzacola lagoons were endosulphan II (250 ng g-1) and
p,p'-DDE (22 ng g-1), both occurring during the dry seasons and heptachlor epoxide (61 ng g-1) during the
rainy season. Predominance of endosulphan can be explained since it is one of the authorized agrochemicals
being used in Mexico.
In Carretas-Pereira Lagoon, the most evident compounds in sediments during the dry seasons were heptachlor
(23 ng g-1) and delta HCH (22 ng g-1) during April 1997, and Aldrin (151 ng g-1) in February 1998. Heptachlor
epoxide was most abundant (113 ng g-1) during the rainy season. The high concentration of this agrochemical
compound occurs because cotton was one of the most important crops in Chiapas, for which organochlorine
compounds, mainly heptachlor, were widely used. Although it has not been used for several decades, traces of
this compound can still be detected due to its high persistence in nature.
It is worthwhile to note that the highest pesticide levels were found during the dry season when high evaporation
rates favoured the concentration of these agrochemical compounds.
In general, the highest levels of organochlorine pesticides in sediments were recorded in the Carretas-Pereira
Lagoon; Heptachlor, heptachlor epoxide, and Aldrin were the dominant compounds. In the Chantuto-Panzacola
system, endosulfan II, p,p'-DDE and heptachlor epoxide were the most abundant compounds. It should be noted
that the use of most of these compounds has been restricted and even prohibited due to their high toxicity. The
observed differences can be accounted for by the different agrochemical compounds used for diverse
agricultural purposes according to the type of crops cultivated around the lagoons and to the amount of
pesticides applied.
When the above result from Botello et al. are comparison with previous studies conducted in the Mexican
Pacific, the total concentrations recorded in the sediments of Chantuto-Panzacola (14-262 ng g-1) and Carretas-
Pereira (18-993 ng g-1) lagoons are far higher than those reported 15 years ago by Rosales et al. (1985) in the
Yavaros Lagoon (1.85-10.45 ng g-1) and the Huizache-Caimanero system (5.1-16.4 ng g-1), both located in
Sonora state.
80
Finally, because of their economic importance to the region, the concentrations of organochlorine pesticides
were determined in white shrimp (Penaeus vannamei) and in the brown porgy (Lutjanus novemfasciatus) from
both lagoons, using a set of 50 shrimps and 20 fishes from each lagoon. In the Chantuto-Panzacola Lagoon, the
concentrations were greater (94 ng g-1) in muscle of the dark porgy (L. novemfasciatus) than those found in
muscle (not detectable) and exoskeleton (21 ng g-1) of the white shrimp (P.vannamei). Furthermore, in the
white shrimp (P. vannamei) muscle from the Carretas-Pereira Lagoon only heptachlor and p,p'-DDE were
detected (5 and 2 ng g-1 respectively), whereas the exoskeleton only showed low delta HCH (2 ng g-1) and p,p'-
DDE (0.5 ng g-1) values. The organochlorine pesticide concentrations in Penaeus vannamei muscle (7 ng g-1)
might result mainly from the feeding habits of these organisms, which allow them to ingest sediment particles
with associated pollutants, such as organochlorine pesticides that accumulate in muscles due to their lipophilic
properties. These concentrations in the white shrimp (P. vannamei) muscle are higher than those reported by
Rosales and Escalona (1983) for the same species from the Caimanero (1.02 ng g-1) and the Moroncarit (1.4 ng
g-1), lagoons in the state of Sinaloa, in the northwest of Mexico.
Another study has been reported concerning the levels of chemicals in mangrove oysters (Crassostrea
corteziensis). The samples were collected in April and May 1996, at 14 mangrove site along the Pacific Coast of
Mexico, extending southward from the state of Sonora (Yavaros) to Barra de Navidad in Jalisco (about 16º north
latitude), a distance of about 1,100 kms. The stations are roughly equally spaced along the coast, from 1.Yavaros
Lagoon (YV) to 14. Barra de Navidad Lagoon (BN). The fourteen stations are identified as follows:
1.
Yavaros Lagoon (YV) (26º 41.5' N)
2.
Agiabampo Lagoon (AG)
3.
Ohuira Lagoon (OH)
4.
Navachiste Lagoon (NA)
5.
Santa Maria Lagoon (SM)
6.
Altata-Ensenada del Pabellon Lagoon (AEP)
7.
Ceuta Lagoon (CE)
8.
Mazatlan Port (PM)
9.
Caimanero Lagoon (CA)
10.
Teacap an-Agua Brava Lagoon (TA) ,
11.
Mexcaltit an Lagoon (MX)
12.
San Crist obal Lagoon (SC)
13.
Nuevo Vallarta (NV)
14.
Barra de Navidad Lagoon (BN) (19º 11.7' N)
High Pb concentrations were found in oysters from the center and southern Sinaloa coast: AEP (7.6 ng/g); PM
(7.4 ng/g); and CE (7.1 ng/g). The lowest concentrations of Pb were found at towards the north and south ends
of the testing region: at SC (<2.3 ng/g) and OH (2.5 ng/g). Since the coefficient of variation for Pb is large there
is a question regarding the statistical significance of these differences.
The lowest concentrations of PCBs were found in non urban areas, AEP (3.4 ng/g), CA (13.4 ng/g) and NV (29
ng/g) while the highest concentrations of PCBs were detected in oysters collected near to suburban centers, NA
(655 ng/g), PM (459 ng/g) and YV (390 ng/g. The main chemical forms of PCB found most frequently were
Aroclor 1242 (87100% of total), Aroclor 1254 (011%) and Aroclor 1260 (010%).
In oyster samples collected ten year s earlier (19861993) on the Mexican Pacific coast, PCBs levels were found
below 100 ng/g (Sericano et al., 1995); Mazatlan and Altata-Ensenada del Pabellon were included in this
sampling. Martin and Gutierrez-Galindo (1989) found PCB concentrations of 6.77.4 ng/g in C. corteziensis
collected during 1988 in Mazatlan harbour. Comparing these results with those obtained here, it is clear that the
situation in Altata-Ensenada del Pabellon is steady, but in contrast, Mazatlan shows an increasing PCB
contamination.
Of 22 organochlorine compounds detected ( as reported in Paez-Osuna et al., 1998), the pesticide with the
highest frequency of occurrence and concentration was HCB, which is used alone or in combination with other
fungicides in mixed seed protectants. Another explanation of its distribution is as a by-product of, and accidental
81
release from, many chemical and pesticide manufacturing processes. HCB results from an impurity during the
synthesis of several herbicides and pesticides.
High HCB concentrations were found in oysters from those lagoons that are bordered by extensive agricultural
lands: YV (911 ng/g) and NA (183 ng/g). Values were low, less than 100 ng g l, at all other sites, with the
lowest HCB concentrations being measured in areas with limited agricultural activities: SC (1.1 ng/g) and BN
(2.0 ng/g).
Lowest concentrations of PAHs were found in oysters from stations TA (120 ng/g), NV (180 ng/g), and AG
(251 ng/g). The highest PAH concentrations were measured at SM (3520 ng/g) and AEP (1820 ng/g). The
relative patterns of abundance of the individual aromatic hydrocarbons can be used to suggest probable sources
of PAHs in bivalves (Farrington et al., 1983); i.e. the presence of phenanthrenes plus fluoranthene indicates a
pyrogenic source for many of the aromatic hydrocarbons. Benzo(k) fluoranthene and benzo(b)fluoranthene were
among the more common and abundant compounds (0100% of PAHs) found in most of the oysters in which
were detected the highest concentrations of aromatic hydrocarbons. In most of the sites sampled, low
concentrations of PAHs were present; fluoranthenes (<27.5% of PAHs) and phenanthrenes (<16% of PAHs)
were in relatively low proportions at these sites.
The most likely sources of PAHs at the latter sites include the aeolian and the fluvial transfer of fossil fuel
pyrogenic residues, transported either from the densely populated surrounding areas (as in AEP and SM) or
from local sources, such as the exhausts of the thousands small boats that are used extensively in fishing
activities in areas including YV, AG, OH, NA, SM, AEP, CE and PM.
3.1.5 Emerging Chemicals of Concern
With the development of regional and global agreements to ban PTSs, the attention of regulators and researchers
has turned to other chemicals with similar properties. The UNEP and the UN ECE lists are not closed. New
substances can be added if they meet the scientific criteria for inclusion and if ratified by the signing parties
(Muir et al. 2001).
The process of identifying new substances is ongoing. An important example is the workshop that was held on
March 6 2001 at the offices of the U.S. EPA Great Lakes National Program Office in Chicago, IL to inform the
relevant state and federal agency scientists of new emerging contaminants occurring in the Great Lakes that
might be of interest to fish monitoring programs. (U.S. EPA Workshop 2001) Some of the results and
conclusions from the workshop follow. Among the substances there are some which have not been mentioned
earlier in this report.
The first three compounds are flame retardants, which were discussed in Chapter 2 (Section 2.2.4.6. and
2.2.4.7). Because of the growing attention individual flame retardants are receiving, further details are provided
here. (M. Alaee and R.J. Wenning 2002)
3.1.5.1 Polybrominated Diphenyl Ethers (PBDEs)
PBDEs have three technical mixtures: penta, octa and deca. Each mixture contains several congeners with the
majority of them being penta, octa or deca, respectively. PBDE mixtures, containing approximately 12
congeners, are not as complex as PCB mixtures. PBDEs are known to have low dioxin-like toxicity, but they are
currently unregulated.
It is important to note that the characteristics of the individual technical mixtures are not the same. For example,
the level of bioaccumulation, the toxicity and the long-range transport characteristics of the technical mixture
are different. However, in the following discussion, reference is occasionally made to PBDEs as a group of
chemicals, where the characteristic of individual technical mixtures are not described separately because they
were not analyzed separately. Hence, care must be taken when considering characteristics ascribed to the group
of PBDEs as a whole.
The lower brominated congeners have a longer half-life in humans. BDE-183 has a half-life of 85 days, while
the BDE-209 has a half-life of 7 days. Bioaccumulation is thought to be more important for smaller congeners,
82
and less so for larger ones such as deca. The deca congener, BDE-209, is likely to be too large to pass through
the cell membranes, which explains why it bioaccumulates less and has a shorter half-life in humans.
A recent Swedish study of human breast milk shows PBDEs exponentially increasing with a doubling time of 5
years (Meironyte et al., 1999). These samples also show an exponential decrease of DDT over time. Recent
studies in the U.S. indicate that human breast milk in North America is also contaminated by PBDEs. Breast
milk of North American women contains the highest level of PBDEs in the world, some 40 times greater than
the highest levels reported for women in Sweden. The levels of PBDEs in North Americans appear to be
doubling every two to five years, according to Mehran Alaee, a research scientist with Environment Canada
Data concerning levels of PBDEs in human breast milk may indicate that PBDEs will replace PCBs/DDTs as
the major environmental POP over the next 15-30 years, especially since PBDEs continue to be manufactured
within the North American region (Hooper, K. and T.A. McDonald 2000; Noren, K. and D. Mieronyte 1998;
and Alaee, M. and R.J. Wenning 2002).
PBDEs have been measured in several media. The most common congeners found in environmental media are
47, 99, 100, 153, and 154 with 47 being the most abundant (congeners have the same numbering system as
PCBs). They have been found in humans, fish, air, and human umbilical cord blood. In Lake Michigan
salmonids, the concentration range is 45-150 ng/g ww for total PBDEs (Manchester-Neesvig et al., 2001). Air
near Eagle Harbor (L. Superior, USA), Sturgeon Point (L. Huron, USA) and Sleeping Bear Dunes (L. Michigan,
NE) has a range of 5-20 pg/m3 (Strandberg et al., 2001). M. Ikonomou reports that PBDEs appear to be
contaminating sections of Arctic rather rapidly with concentrations of PBDEs doubling in ringed seals every
five years. (Ikonomou et al. 2002)
In addition, temporal trend data for PBDEs in gull eggs from Snake Island between 1981 and 1999, and lake
trout from Lake Ontario showed a 300-fold increase in PBDEs between 1978 and 1998 (Muir et al. 2001).
The air in Chicago has PBDE concentrations of 40-70 pg/m3 (U.S. EPA Workshop 2001).When analyzing for
PBDEs in air, it is important to measure both the concentration on particles and the concentration in the vapour
phase. Gas-particle partitioning studies have shown that 20% of the lower molecular weight PBDEs are found
on particles whereas the 80% of the larger PBDEs are found on particles (Strandberg et al. 2001).
Due to their toxicity, widespread use, and demonstrated occurrence, it was concluded that PBDEs should be
analyzed in the Great Lakes Fish Monitoring Program.
Furthermore, breakdown products of PBDEs like mixed bromo/chloro dioxins/furans are much more toxic than
PBDEs. The transport and fate of PBDEs should be studied to identify the breakdown products of these
materials. Little has been reported on other related materials like chlorinated diphenyl ethers (PCDEs). In many
areas and matrices, e.g. Great Lakes fish, they can be as high or higher than PBDEs.
3.1.5.2 Polybrominated Biphenyls (PBBs)
PBBs are flame retardants used in plastics. They have been banned in the U.S. since 1977 because of their
toxicity. Worldwide production of these chemicals stopped last year. Despite having been banned, PBB-153
continues to be found in human blood in Michigan. This may be due to a 1973 accident in Michigan in which
PBBs (mostly 153) were accidentally mixed with dairy cow feed. This accident caused PBBs to get into the milk
supply and be widely distributed and consumed in the state. It was recently reported that PBB-153 is found in
lake trout from all the Great Lakes (Luross et al., 2001). The concentrations have decreased from 30 ng/g to 15
ng/g over the period 1978-1988, but it is still present and may be of concern in fish. (U.S. EPA Workshop 2001)
3.1.5.3 Tetrabromobisphenol A (TBBPA)
Another emerging contaminant was TBBPA. Worldwide production is estimated to be 1.2 x 108 kilograms,
which is greater than PBDE production. It is used mainly as a reactant to make polymers and resins (Personal
Communication, American Chemistry Council 2002). It is mostly used in computer circuit boards as a flame
retardant. TBBPA has been found in sewage sludge in Canada and in blood plasma in Sweden. Sediments in
Japan and Sweden have also shown measurable concentrations of TBBPA. There have been no attempts to find
TBBPA in fish. In a sludge sewage treatment plant, TBBPA concentrations ranged from 1-130 ng/g. Most of the
samples have been in the range of 20-60 ng/g. (U.S. EPA Workshop 2001). ). The American Chemistry Council
83
reports that TBBPA is not used in polyurathane foam and that there is a minor application of this product as an
additive-type flame retardant for thermoplastic ABS polymers. (Personal Communication, American Chemisty
Council 2002).
TBBPA is not extracted from polyurethane foam using conventional methods. The methods used for
"conventional" persistent, toxic substances such as PCBs and organochlorine pesticides are not effective for
TBBPA. Also, one must consider what media to analyze. To find TBBPA in fish, the blood and liver should be
analyzed. TBBPA is potentially toxic and may be a good candidate for the fish monitoring list after methods
have been improved, and evidence has shown that TBBPA is a concern in fish. TBBPA was added to the US
EPA TRI list of substances in the last few years. (U.S. EPA Workshop 2001)
3.1.5.4 Short Chain Chlorinated Paraffins (SCCP)
Sewage treatment plant effluent, sediments, water, and fish around Lake Ontario have been analyzed for these
compounds. Concentrations in water have been found in the ng/L range. In fish, 100 ng/g ww has been detected.
One of the highest concentrations found is in Fox Lake surface sediment in the Yukon at 250 ng/g. Analytical
methods are difficult due to the complexity of the SCCP mixture. (GLNPO 2001)
3.1.5.5 Perfluorooctane Sulfonate (PFOS)
The class of chemicals called fluorinated surfactants have been generating growing concern. The focus has
fallen mainly on PFOS. This chemical is used in the manufacture of numerous products and is thought to be the
degradation product of several other electrochemically fluorinated surfactants. Fluorinated compounds produced
by telomerization are not thought to degrade to PFOS. As discussed in Section 2.2.4.12., the uses of fluorinated
surfactants are numerous and diverse. (U.S. EPA Workshop 2001)
The presence of PFOS and perfluorooctanoic acid (PFOA) in liver samples of top predators, such as polar bears
and fish-eating birds, as well as in human blood samples, was recently reported by the manufacturer, 3M
Company. These compounds are anions and have low octanol-water partition coefficients. However, they persist
in biota because of the great stability of the perfluoro group and because they are recycled in the entero-hepatic
circulation. This new bioaccumulation pathway may be important for a wide range of chemically stable, polar
compounds, and this raises the issue of whether criteria for identifying PTSs will have to be modified (Muir et
al. 2001).
The recent OECD report concludes that PFOS is persistent in the environment and has been shown to
bioconcentrate in fish. It has been detected in a number of species of wildlife, including marine mammals. Its
persistence, presence in the environment and bioaccumulation potential indicate cause for concern. It appears to
be of low to moderate toxicity to aquatic organisms but there is evidence of high acute toxicity to honey
bees(OECD 2002a).
The following table shows some current representative concentrations of PFOS in the environment (Giesy and
Kannan, 2001; Kannan et al., 2001).
Table 2: Current Representative Concentrations of PFOSs in the Environment
Animal
Location
PFOS concentration
Units
Ringed Seal Plasma
Can. Arctic
<3-12
ng/mL
Baltic Sea
16-230
ng/mL
Gray Seal Plasma
Can. Arctic
11-49
ng/mL
Baltic Sea
14-76
ng/mL
Polar Bear Liver
Alaska
180-680
ng/g
Polar Bear Blood
Alaska
26-52
ng/g
Mink Liver
Michigan
590-4900
ng/g
Herring Gull blood
Great Lakes
66-79
ng/mL
Herring Gull plasma
Great Lakes
277-453
ng/mL
Cormorants L.
Huron
340
ng/L
84
Animal
Location
PFOS concentration
Units
Cormorants L.
Superior
92
ng/L
Bald Eagle Plasma
Midwest US
2-2570
ng/mL
Albatross sera
Pacific Midway Is.
3.5-20
ng/mL
Cormorant egg yolk
Washington DC
24-254
ng/g
Chinook fish liver
Great Lakes
110
ng/g
Comparisons can be made between different animals in different locations, concentrations in different parts of
the body, spatial differences and differences between trophic levels. In dolphins, measurements were taken to
ascertain if there were significant concentration differences between male and female and their ages. No
significant differences were found. Toxicity studies show that PFOS interferes with fatty acid binding and
transport, decreases cholesterol, inhibits cell-to-cell communication, potentially causes cancer, and has
reproductive and developmental effects (Hu et al., 2001). Human blood levels have been published recently by
Hanson et al. (2001). Future research is needed on this chemical because it is ubiquitous and persistent. It is
lipophobic unlike other persistent, bioaccumulative toxic chemicals, and found mostly in the blood and liver.
Although the recent OECD assessment (OECD 2002b) has contributed considerably to our understanding of
PFOS, further studies are required on the toxicology, fate, transport and bioaccumulation potential of PFOS.
(U.S. EPA Workshop 2001)
3.1.5.6 Polychlorinated Naphthalenes (PCNs)
PCNs and substance groups in the following three subsections have not been discussed earlier in this
assessment.
In the past, PCNs were used in the electrical industry because of their high thermal stability and inertness. PCNs
were also a contaminant in PCB technical mixtures. Production of these chemicals in the U.S. and Europe
ceased in the 1980s. Global production has been estimated at about 1.5 x 108 kilograms. In Great Lakes fish,
PCN concentrations of 19-31,400 pg/g ww have been found (Kannan et al., 2000). TEQs range from 0.007 11
pg/g ww. Because of their dioxin-like toxicity and bioaccumulation potential, PCNs are good candidates for
addition to Great Lakes fish monitoring programs. (U.S. EPA Workshop 2001)
3.1.5.7 Alkylphenol Ethoxylates (APEs)
APEs are used as surfactants in soaps and detergents. Approximately 1.8 x 108 kg/yr are used in the US. Sixty
percent of this enters the aquatic environment and can mostly be found in sediments. Difficulties with the
analysis of APEs include numerous isomers, branched chains and poorly developed methods. These chemicals
are endocrine disrupters. The nonylphenol ethoxylates are the most widespread of the APEs. Nonylphenol (NP),
nonylphenol monoethoxylate (NP1EO), and nonylphenol diethoxylate (NP2EO) are all detected degradation
products (U.S. EPA Workshop 2001).
Bottom feeders such as carp have been found to have higher concentrations of APEs than pelagic fish. Detroit
large mouth bass have been measured with concentrations of 0.1-0.4 µg/g ww. The mean of six Chicago River
carp was 13 µg/g ww, with a range from 7 33 µg/g ww. In order to get an accurate assessment of exposure,
NP, 1EO and 2EO all must be measured. In carp from contaminated sites, the ratio has been found to be
approximately 1:1.7:1 whereas in lake trout from Sturgeon Bay, 80% of the total APE concentration is NP.
APEs should be considered for future inclusion in the fish monitoring program for near-shore waters but are not
thought to be relevant for open lake monitoring (U.S. EPA Workshop 2001).
3.1.5.8 Current-use Pesticides
Dachtal and chlorothalonil are common pesticides in current use. Both have been found in Great Lakes air and
precipitation at concentrations as high as or higher than other organochlorine pesticides measured by IADN
(Hites and James, 1999). Chlorothalonil is used on peanuts and potatoes as a fungicide. It is potentially toxic and
bioaccumulative and should be looked for in fish. (U.S. EPA Workshop 2001)
85
3.1.5.9 Pharmaceuticals and Personal Care Products (PPCPs)
A new area of research has focused on pharmaceuticals and personal care products in the environment. Sources
of these include animal feedlots, wastewater treatment facilities, septic systems, and medical industry
discharges.
The United States Geographical Survey (USGS) is currently conducting a national survey of these contaminants
in selected watersheds. One hundred at-risk streams in 24 states were sampled. The typical concentration range
of chemicals found was 0.01-1.0 µg/L, with most concentrations below 0.1 µg/L. The most abundant substances
were insect repellents, non-prescription drugs, detergents, antibiotics, caffeine, and cotinine, a by-product of
tobacco. Benzalkonium chloride (BAC), an antiseptic and antimicrobial kitchen cleaner, has been found in
sediments and is a strong candidate for bioaccumulation. Difficulties with measuring pharmaceuticals and
personal care products are that many of the degradation products are unknown, drug compositions constantly
change and there are currently few data on environmental levels. Implications of these substances being in the
environment include human and ecosystem health, unknown cumulative and synergistic effects, and
development of antibiotic resistance. It is possible that some of these chemicals may be able to be used as
environmental tracers. The toxicity and environmental chemistry of most PPCPs are not well known. Future
research in this area should seek to improve the methods and determine concentrations in sediments and tissue.
(U.S. EPA Workshop 2001)
3.1.5.10 Halogenated Phenolic Compounds (HPCs)
Recent studies have found that halogenated phenolic compounds (HPCs), including pentachlorophenol (PCP),
hydroxy-substituted PCBs (OH-PCBs) and hydroxy-brominated diphenyl ethers are present in the blood of
marine mammals and humans in Canada from the Arctic and temperate regions of North America. HPCs have
been shown to have endocrine disruption potential based on in vivo and in vitro assays, while OH-PCB sources
may be primarily from metabolism of other PCB sources. (Muir et al. 2001, Kester et al. 2000)
3.2 Ecotoxicology of PTSs of Regional Concern
Pollution is principally a biological problem in that its primary effect is on organisms. Yet, most pollution
assessments emphasize the measurement of chemical and physical variables rather than responses of organisms
to these variables. In Section 3.1, the information available on observed effects is sparse compared with the
material presented on levels and trend in ecosystems. There are several reasons for this contradictory emphasis
on physiochemical variables, but perhaps the most compelling is the lack of predictive information on responses
of organisms, singly or in association, to specific environmental factors. There is a need to further the
development of methods for objectively defining relationships among biological and physiochemical variables
in environmental ecosystems (USGS 2002)
This situation is perhaps acceptable in the present instance for two reasons. First, the purpose of the UNEP RBA
is to provide support to policy development. From the perspective of managing chemicals of interest and
ameliorating their impacts, the environmental information required to make policy decisions could be less
stringent than that required by the scientific community which is striving to develop an understanding of the
complex inter-related responses of the whole ecosystem to the full suite of chemicals. Second, the policy
decisions will focus heavily on the human health implications of PTSs.
The intention in this section is to provide a sense of the way PTSs behave in the environment. The individual
substances with their widely varying ecotoxicological characteristics are not addressed. The emerging chemicals
of concern are not mentioned at all. Such discussions would not be appropriate in this overview assessment.
In the following subsection the uptake, accumulation, storage and disposal of PTSs by biota is described. The
focus is on terrestrial and aquatic ecosystems. The next subsection is a general description of the possible effects
that contaminants can produce. The final subsection focuses on the national and international work underway to
identify and document endocrine disrupting chemicals.
3.2.1 Toxicokinetics
Since the toxicokinetics of organometals are substantially different from the other PTSs, these two groups of
PTSs are discussed separately. There is an Arctic bias in the following discussion since the AMAP Assessment
86
report provided much of the material in this section (AMAP 1998). The original references have been retained
here to facilitate access to the source literature.
3.2.1.1 Non-metalic PTSs
Uptake and Accumulation
The majority of the non-metalic PTSs dealt with in this assessment are lipophilic, stable, and persistent. They
are taken up by aquatic and terrestrial fauna via diffusion over the gills and from food in the gastrointestinal
tract. Non-metallic PTSs, particularly organochlorides (OCs), cross the gill/gut membrane and enter the blood
where they are quickly distributed to high lipid tissues such as the liver and adipose tissue. Metabolism and
elimination are often slow, leading to a net increase of these substances in the organism over time.
As a consequence of persistence and bioaccumulation, PTSs can accumulate to relatively high concentrations in
biota at low environmental exposures, as has been shown in the case of Great Lakes (Swain et al., 1993) and
Lake Baikal (Lebedev et al. 1998; Poliakova et al., 2000). In the aquatic environment the primary root of initial
entry into the food chain is through active uptake of PTS-contaminated particulate matter by filter-feeders and
plankton (Thomann et al., 1992) while at higher trophic levels, through dietary uptake rather than direct
absorption.
Species Differences
There are species differences in the tissue distribution of OCs, partly due to differences in lipid distribution. For
example, high concentrations of orally administered 2,3,3',4,4'-PeCB (CB105) were found in the liver and brain
of cod, while rainbow trout accumulation was in the extrahepatic fat depots (Bernhoft et al. 1994). Lipid
dynamics can also affect the distribution of OCs. Female kittiwakes (Rissa tridactyla) showed a redistribution of
PCBs from the liver and body fat to the brain during the period of prebreeding to late chick rearing. This was in
part due to the mobilization of lipids from the liver and body fat during reproduction and subsequent loss in
body mass, which in turn led to higher lipid weight PCB concentrations in the remaining lipids (Henriksen et al.
1996). These examples imply that different tissues in different species will be the targets for possible effects
from OCs, and this in turn is affected by lipid distribution and dynamics.
Concentrations
According to Bergman (2002), in-depth studies have been performed to describe the fate and effects of a rather
limited number of PTSs , namely DDT/DDE, PCBs and PCDDs/PCDFs ("dioxins"). It is only for these
pollutants that it is possible to make temporal and geographical comparisons and to determine bioaccumulation
and biomagnification properties. The highest PTSs concentrations (ppm level) have been reported for DDE and
PCBs, levels of 5-6 orders of magnitude higher than those reported as TEQs for PCDDs/PCDFs, (ppt level). In
the intermediate range of concentrations we have other PTS such as HCB, Chlordane, Mirex and Toxaphene. It
is difficult to determine the effects of these latter chemicals since their effects, as indicated in toxicological
experiments for example, are hidden by the effects produced by PTS present at the highest concentrations and/or
the effects of the most toxic pollutants.
Metabolism
Metabolism of xenobiotics occurs mainly in the liver via a two-phase process. In phase I, xenobiotics are
converted by oxidation reactions to metabolites that can undergo phase II reactions. In phase II, the product is
conjugated with glucuronic acid or glutathione, for example, to produce water-soluble compounds that can be
excreted in urine or bile. These processes are catalyzed by liver enzymes such as the cytochrome P450
containing monooxygenases (Nebert and Gonzalez 1987). Substances that are resistant to metabolism will be
selectively accumulated in living organisms. In addition to detoxification, the enzymatic processes can also
create reactive intermediates that may be mutagenic and/or carcinogenic, or metabolites that are lipophilic and
have retained toxicity or that have the ability to bind selectively to proteins and accumulate in the organism.
Many organochlorines (OCs) form metabolites that are biologically active. DDT is metabolized in living
organisms to DDD and further to DDE, both of which are lipophilic and toxic and accumulate in biota (WHO
1989a). In some cases, a methylsulfone (MeSO2) group is added during metabolism and a number of MeSO2-
DDE and MeSO2-PCB congeners have been identified in animals (Jensen and Jansson 1976, Lund et al. 1988,
Haraguchi et al. 1990, 1992, Bergman et al. 1992b, 1994b, Brandt et al. 1992, Letcher et al. 1995 and 1995b,
87
Polischuk et al. 1995). Some congeners of PCB may also form hydroxylated metabolites (Jansson et al. 1975).
This type of metabolite has been found to selectively bind to transthyretin, one of the major transport proteins
for retinol and thyroid hormones in the blood (Brouwer et al. 1988, 1990, Bergman et al. 1994a, 1992a). Aldrin
is metabolized in living organisms to dieldrin by the cytochrome P450-dependent monooxygenase, aldrin
epoxidase (WHO 1989b). -chlordane is metabolized to some extent to oxychlordane (WHO 1984).
Hexachlorobenzene is metabolized to some extent, mainly by the liver, and may form, among other metabolites,
pentachlorophenol, tetrachlorohydroquinone, pentachlorothiophenol and lower chlorinated benzenes (Debets
and Strik 1979, Renner 1988).
Excretion
The major excretion route of organochlorines and their metabolites is via the feces. Some of this is passive
diffusion over the gut membrane and some from bile excretion of metabolites. In invertebrates and fish,
excretion also occurs by diffusion over the gill membranes. Female fish and birds excrete lipophilic
organochlorines via their eggs, and female mammals via placental transfer to the fetus and in breast milk. As a
consequence of the transfer to the young, in the Arctic, young harp and hooded seals can have as high levels of
some OCs as their mothers at the end of the lactation period (Espeland et al. 1997) and young polar bears (1-2
years) have similar PCB levels to adult females with high PCB levels (Bernhoft et al. 1997), and polar bear
cubs-of-the-year have higher concentrations of many OCs than their mothers (Polischuk et al. 1995). This is of
concern as young animals may be more sensitive to the effects of OCs than adults.
Mixtures of OCs
The net result of uptake, distribution, metabolism, and excretion will determine the OC levels found in an
organism. The result however, is affected by other factors. Studies carried out to determine the uptake,
distribution, metabolism, and excretion of OCs are usually done with one substance at a time. This approach is
unrealistic since wildlife and humans are exposed to complex mixtures of OCs. Very little is known about how
different OCs affect each other's toxicokinetics. OCs that induce the hepatic cytochrome P450 system will affect
the metabolism of other xenobiotics, which may lead to an increase in xenobiotic metabolism, thus increasing
excretion. For example, studies on Baltic seals show that high body burdens of DDT and PCB are associated
with lower relative amounts of the mono-ortho CB, 2,3',4,4',5-PeCB (CB 118). At PCB concentrations (sum of
CBs 28, 52, 101, 118, 138, 153, 181) of 50 µg/g lw or higher, CB 118 could not be found (Haraguchi et al.
1992, Olsson et al. 1992b). PCB levels in Arctic ringed seal are much lower, xenobiotic metabolism does not
seem to be induced, and concomitantly, CB 118 is present in higher relative amounts (Norstrom and Muir 1994).
It has been shown that mink exposed to both DDT and PCB only biomagnify PCB whereas mink exposed either
to DDT compounds or PCBs biomagnify the two groups of compounds at a similar rate (Kihlström et al. 1992).
An increase in xenobiotic metabolism may also lead to an increase in the formation of reactive intermediates,
with increased toxicity and tissue damage (Boon et al. 1992). There are indications, for instance, that PCB
exposure may influence the magnitude of carcinogenicity of PAHs in fish (Bailey et al. 1989) and that exposure
to PCBs also increases the uptake of PAHs in English sole (Stein et al. 1984).
Biological Variation
Xenobiotic metabolism is also subject to biological variation. In a study of salmon (Salmo salar), the
cytochrome P450 enzyme system was followed for a year and showed cyclical variations in enzyme activity.
The basal enzyme activity measured as ethoxyresorufin-O-deethylase (EROD) levels was higher in both males
and females during the winter months and then dropped during the summer, most particularly during the period
of sexual maturation (Larsen et al. 1992). Significant differences were seen in EROD levels between males and
females at sexual maturation, with females having lower or non-detectable activity just before ovulation.
Thus, it is very difficult to evaluate the toxicokinetics of environmental exposures to mixtures of OCs. The
interactions that have been identified indicate that the relative amounts and the composition of various
contaminants in animals may partly be the result of selective effects on the organism's uptake, metabolism, and
excretion of OCs, and not solely a result of the specific pollution burdens of contaminants in the area.
88
Laboratory and Manipulated In Situ Studies
Although a wide range of laboratory and manipulated in situ studies with various organisms have been
documented in the literature, the results should be carefully evaluated. On one hand, the controlled laboratory
toxicological studies with individual compounds or carefully prepared mixtures permit a clearly-defined dose-
response causality between chemical exposure and observed effects. On the other hand, the laboratory tests
alone seldom adequately describe what is likely to occur in the environment. The often complex and subtle
effects of chronic, low-level environmental exposure to PTS are simply not well understood. In the environment,
the exposure of organisms to low level mixtures of several chemical contaminants is common. In such
conditions it is extremely difficult to ascribe an observed effect to any particular contaminant. There is also the
possibility that, in the environment, toxic substances in combination may act additively, antagonistically or
synergistically.
Since the results of manipulated toxicity tests can significantly differ from the ecological reality, the results in
this chapter have been based preferentially on reported field studies and observations, as much as possible. Of
course the field information available is far from adequate and in some important areas, entirely absent.
3.2.1.2 Organometals
Uptake and Accumulation
Much of the Hg in the environment is unavailable to organisms, as it is strongly bound to sediment or organic
material. Inorganic forms can be methylated by micro-organisms and transformed to methylmercury, which is
much more readily taken up and accumulated in both aquatic and terrestrial organisms.
In general, invertebrates and fish take up only a small proportion (0.1% for Cd) of the metals in the water
through the gills while intestinal intake is larger; the most efficient intake is for methylmercury (70-80%).
Environmental variables such as temperature, pH, and redox potential are particularly important for Hg uptake
in fish. Data on uptake suggest that absorption increases with higher temperatures and lower pH.
For higher organisms, metals are efficiently (7-94%) absorbed through the lungs, with the degree of absorption
varying by animal species, metal, and chemical form of the metal. Mercury vapour and H2SeO4 are taken up
most efficiently (80-94%), but Pb and Cd readily cross the lung epithelium (7-50%). The uptake through the
intestine also depends on the organism, metal type, and chemical form. Only 1-16% of Pb and Cd are taken up
by various species, whereas 57-95% of Hg and Se are absorbed through the intestine. Methylmercury is taken up
more than six times as efficiently as inorganic Hg.
The primary uptake route of Hg in marine and terrestrial mammals is though diet. This is related to the relatively
high concentrations of methylmercury in food items (e.g., fish), which is more effectively taken up than
inorganic forms.
For many animals, it is not known for certain whether Pb is absorbed through the skin or actually taken up via
inhalation or contaminated food. Accumulation in mussels (Mytilus edulis) has been demonstrated to occur in
all tissues, but highest concentrations are seen in the kidney. In fish, Pb accumulates primarily in the gill, liver,
and kidney, though it is not known whether accumulation in the gills represents uptake into the tissue or
absorption onto exterior surfaces. Birds dosed with lead shot show signs of tissue accumulation in liver, muscle,
and bone, and appear to be influenced by the amount of fiber in their diet. Lead also accumulates in eggs and
embryos.
The ability of organisms to accumulate metals to concentrations of one or more orders of magnitude greater than
concentrations in their food usually represents the major pathway leading to chronic toxicity. However,
accumulated metal may be present in tissues in a relatively non-toxic or inert form even if it was originally
toxic, because the toxicity of the metal can be modified through interactions between metals or through
biotransformation by the organism. In contrast to non-metallic PTSs , which are highly lipophilic and therefore
accumulate primarily in body lipids, heavy metals are preferentially accumulated in proteinaceous tissues. The
degree to which metals are accumulated varies greatly depending both on the metals involved and on the organ
or tissue.
89
Once absorbed, heavy metals are distributed in the body by the circulatory system, irrespective of their chemical
form (Foulkes 1988). The fraction of transported metal absorbed by various organs and the fraction
subsequently excreted vary greatly for each metal. The mechanisms involved in selective metal uptake in organs
are not well understood (Foulkes 1988). Metals are initially distributed to a variety of organs and tissues, and
subsequently redistributed to other tissues for storage and inactivation.
Tissue concentrations of Hg increase with age for both marine and freshwater fish. Mercury accumulated in fish
is usually in the form of methylmercury, whereas the source is usually inorganic.
Fish and mammals at higher trophic levels are consumed by humans. These animals accumulate relatively high
levels of methylmercury in kidney, liver, and muscle. Although knowledge of these concentrations is important
for assessing the potential exposure of human consumers to methylmercury, in terms of evaluating the toxic
effect to mammals the most important concentration is that in the brain, which is generally not measured.
Transformation
The formation or breakdown of metal-carbon bonds or a change in the oxidation state of a metal within an
organism (biotransformation) will affect the chemical activity of heavy metal compounds, and therefore their
toxicity. Changes in the oxidation state influence the ability of a metal to interact with various tissue ligands.
Hg, for example, exists in three oxidation states: elemental (Hg(0)), the mercurous ion (Hg2(II)), and the
mercuric ion (Hg(II)). Hg(0) easily penetrates biological membranes because of its high lipid solubility. The
mobility of Hg2(II) and Hg(II) are much more restricted due to their tendency to form salts and their high
affinity for sulfhydryl groups on proteins (Clarkson 1986).
Two biotransformation processes are important to the toxicity of metals:
1. Methylation/demethylation of certain heavy metals and metalloids (e.g., As, Hg, and Se). In some cases,
cleavage may serve as a detoxification pathway, whereas in others the metabolite is the more toxic species.
For example, methylation (the formation of metal-carbon bonds) of inorganic As and Se has been seen to
lead to reduced toxicity in a number of animals and to form the basis for excretable metabolites (e.g.,
methylated selenides), while the reverse is true for Hg. In the case of methylmercury exposure, processes of
demethylation are important for detoxification. In the case of Se, biotransformation in the liver seems to be
the major mechanism by which homeostasis is maintained.
2. Formation of inert complexes also plays an important role in heavy metal detoxification. The biochemical
relationship between metallothionein and such heavy metals as Cd, Zn, Cu, and Hg, for example, is
fundamental to their toxicity. Metals binding with metallothionein form inert complexes, which can be
retained in body tissues (Clarkson 1986). Similarly, Se can reduce the toxicity of certain metals such as As,
Cd, and Hg by forming inert compounds (Högberg and Alexander 1986), which usually accumulate within
organisms.
Excretion
The most important excretory pathways for metal compounds in animals are gastrointestinal and renal.
Gastrointestinal excretion includes excretion of metals into bile (and pancreatic fluid) and excretion by the
intestinal mucosa. Excretion of most organic metal compounds occurs primarily by the bile, while inorganic
compounds are excreted in the gastrointestinal tract. Considerable quantitative differences have been reported
for different animal species. Metals excreted in the bile may be reabsorbed farther down the intestinal tract, and
subsequently re-excreted into bile. Intestinal re-absorption can be prevented if excreted metals form bonds with
non-absorbable compounds. In this way a net gastrointestinal excretion can occur, with the heavy metals being
eliminated from the body with feces. The other mechanism of gastrointestinal excretion involves the removal of
metal compounds in association with the rapid turnover of cells of the intestinal mucosa. Small quantities of
certain heavy metals (e.g., inorganic Cd and Hg) are eliminated from the body when the cells are shed.
Urinary excretion is probably the second most important excretory route for animals. The glomerula membrane
acts as a filter, allowing only those molecules with relatively low molecular weights to pass through into the
renal tubules. Thus, metals bound to low-molecular weight proteins such as insulin or metallothionein may be
cleared from the blood plasma in this way, although a proportion of this is subject to re-absorption. For example,
90
Cd bound to metallothionein is efficiently reabsorbed, with only a small proportion being ultimately excreted
with the urine.
Excretion by invertebrates and fish compared with higher trophic levels is rather fast, with biological half-times
of 3-40, 2-63, and 53-323 days for Pb, Cd, and Hg, respectively. This means that the major route of metals is
through food, where the levels also are higher than in the surrounding water.
In the case of birds, excretion appeared to be enhanced by egg laying, with concentrations of methylmercury
occurring in the egg white and other Hg compounds typically in the yolk. Overall, inorganic forms are more
rapidly excreted than methylmercury.
Lead and Se are readily excreted, which results in moderate levels of these metals in internal organs. Lead,
however, can be deposited in bone, where its half-time can be up to 20 years. Cadmium is excreted slowly once
it is taken up by organisms, with a half-time of 10-50% of the organism's life span (up to 30 years), whereas Hg
has intermediate half-time (12-1000 days). Differences in half-time between the different species partly explain
the generally higher observed metal levels in longer-lived species. The different half-times also explain
differences between the observed levels of the different metals in the various tissue compartments.
3.2.2 Possible Effect
Many of the potential effects of individual PTSs have been described in the literature (AMAP 1998; PANNA
2002a). Table 3 is a matrix of the possible effects that can be produced by some of the principle chemicals in the
UNEP list. The scientific confirmation of these effects in field studies have been limited as has been noted
earlier in this chapter.
Table 3: Potential Effects of Individual PTSs
d
nd
a
ane
l
fan
n
s,
s, an
s
r
d
phene
x
D
F
E
l
drin
T
B
H
D
ibutyltin
Types of Effects
Aldri
die
Chlo
DD
Toxa
Mire
HC
PCD
PCD
PCBs
HC
Endosu
PB
Tr
Reproduction and
U U U U U U U U U U
development
Cytochrome P450 system
U U U U U U U U U
Porphyria
U
U
Immune system
U U U U U U U U U U
Adrenal effects
U U U
Thyroid and retinol effects
U U U U U U
Mutagenic
Carcinogenic effects
U U U U U U U U
Skeletal changes
U
U
The PANNA pesticide database includes information from national and international sources on about 6,000
pesticide active ingredients and their transformation products. The list of chemicals is not strictly limited to
pesticides but includes industrial chemicals. Potential effects include: carcinogenicity, acute toxicity,
reproductive and developmental toxicity, endocrine disruption, neurotoxic cholinesterase inhibitors and
ecotoxicity. The information is most complete for pesticides registered for use in the United States.
3.2.3 Endocrine Disrupting Chemicals (EDC)
· The screening for EDCs for inclusion in the PANNA data base is in progress (PANNA 2002b).
91
Section 3.1.2.2. described the special study of Lake Erie under the LaMPs. Impairments were assessed using
epidemiological criteria, species-specific chemical criteria for tissue concentrations associated with reproductive
effects in field and (or) laboratory studies (Grasman, K.A. et al. 2002). The results are summarized in Table 4.
92
Table 4: Summary of Animal Deformity or Reproduction Impairment with the Likely Cause
Species/
Reproduction
Deformities
Physiology
Notes
Species
Impaired?
Likely
Impaired?
Likely
Impaired?
Likely
Type of
Group
Cause
Cause
Cause
Impairment
Bald Eagle
Yes; observed; PCBs,
Yes; observed
PCBs
No data
* Extent of impairment is probably
exposure
dieldrin,
obscured by hacking/fostering and
above effect
DDE
immigration from less contaminated
levels
inland territories
Colonial
Yes; observed PCBs,
Yes; observed; PCBs
Yes; observed; PCBs, other
Immune
* Most data from W. basin and
Waterbirds
in Herring
possibly
exposure above
exposure
organochlorin system,
Herring Gulls eggs?
Gull; exposure other
effect levels
above effect
es (OCs)
reproductive
* Tree nesting cormorants hard to
above effect
chemicals
levels
organs,
study, but contaminant concentrations
levels in
thyroids, liver are among highest in Great Lakes and
Herring Gull,
enzymes,
are likely associated with embryonic
cormorant and
vitamin A and mortality and deformities?
Common Tern
porphyrins*
* Cause of recent reproductive failures
eggs
of Herring Gulls on W. Sister Is. may
include PCBs, microcystin and (or)
other factors
* Although Caspian Terns have
attempted to colonize Lake Erie as
recently as 1996, they are still too rare
in the basin for field study.
Tree Swallow No
No data
No
* Significant OC exposure; resistance
to effects may make swallow a poor
indicator species for other insect-
eating songbirds
Mink
Likely; PCB
PCBs
No data
No data; likely,
levels in food
based on PCB
above effect
levels in food
levels
Otter
Insufficient
PCBs
No data
No data
* Too rare in Erie basin for study, as
data, but likely
they were re-introduced in 1986.
based on
predicted high
exposure
Snapping
Not observed, PCBs, other Not observed,
PCBs,
Likely organochlorin Endocrine /
Turtle
but exposure at OCs
but exposure at other OCs
es
reproductive
some Ohio
some Ohio sites
sites above
above effect
effect levels
levels
93
Species/
Reproduction
Deformities
Physiology
Notes
Species
Impaired?
Likely
Impaired?
Likely
Impaired?
Likely
Type of
Group
Cause
Cause
Cause
Impairment
Frogs/ Toads Likely
High DDE
No data
No data
* Nitrate concentrations in Lake Erie
and nitrates
watershed often exceed lethal and
sublethal concentrations for
amphibians in laboratory experiments
(see section 7.6.4.1.2)
Mudpuppies Considered
Yes; observed
PAHs and No data
* Data from the Grand River in Ohio
likely (due to
OCs, are
and elsewhere in the Great Lakes
TFM) by the
possible
indicate acute mortality following
authors but
causes
TFM application for lamprey control
inconclusive
(see section 7.6.4.2)
by the LaMP
committee
* porphyrins - the liver synthesizes heme, which is important for hemoglobin and some enzymes. PCBs and other organochlorines block this process and cause the
accumulation of intermediate products called highly carboxylated porphyrins.
94
3.3 Toxicology, Human Levels and Health Effects
Methods for chemical risk assessment (human, environmental and ecological) have been developed at the
international level (IPCS 2002). Risk is the likelihood that harm from a particular hazard will be realized. It is a
function of both hazard and exposure.
Exposure assessment is a complex and developing subject. Essentially, human exposure can occur through
direct exposure (occupation, consumer products) or indirect exposure through the environment (air, water, food,
soil). PTS can enter the human body through both direct and indirect exposure by inhalation, dermal absorption,
or by ingestion. Consideration of these factors gives rise to calculated external exposure, but to relate this to
potential deleterious effects, one must take into account factors such as absorption and metabolism in order to
estimate internal exposures at the target systems, organs or cells.
In this section, we have chosen to concentrate on biological indicators, as a proxy for internal exposure for
human exposure. In this context, the term biological indicator is used to mean a measurable entity found in a
biological material that can be used as a predictor of a potential future health-related outcome. These are usually
indicators of exposure (in the case of PTS, this represents an integrated exposure) although there are other
indicators to assess sensitivity and harmful effects. These indicators use biomedical testing or the measurement
of a chemical (analyte), its metabolite, or another marker of exposure in human body fluids or tissues in order to
estimate and validate human exposure to a hazardous substance (ATSDR Glossary).
Unless otherwise indicated, reference is to potential exposure in North America.
3.3.1 Blood, serum and plasma
There have been numerous studies of levels of DDT and metabolites in human serum and plasma summarized in
Table 5-4 of the ATSDR ProFile document (ATSDR 2000a). Human blood levels of DDT and DDE correlate
with the consumption of fish. The mean serum DDT levels in recreational fishermen around the Great Lakes
decreased by 65% during the period from 1982 to 1989, while corresponding lesser decreases (41%) were also
observed in the control group (Hovinga M., et al. 1992).
In 1982 it was reported that average serum DDE concentrations in the US population were substantially less
than 15 µg/L (Stehr-Green PA 1989).
The average level of DDE, a major break down product of DDT, in the blood (serum) of women with breast
cancer was 562.5 ng/g compared to 505.5 ng/g in the hospitalized women without breast cancer (López-Carrillo,
L., et al. (1997). In another study, the average levels of DDE in the blood (plasma) of 236 women with breast
cancer was 6.01 ppb compared to a slightly higher average level of 6.97 ppb in the 236 women without breast
cancer ( Hunter, D. J., et al. 1997).
ATSDR, which has summarized PCB serum levels in the general population in studies conducted from 1973 to
1996 (ATSDR 2000b), reports current mean serum PCB levels ranging from 0.9 to 1.5 ng/ml in individuals that
do not have a diet high in fish.
Children with cord serum PCB levels of 5.0 ng/mL or more weighed 1.8 kg [4 pounds] less on average than the
lowest exposed children (Ernst Knobil et al., 1999).
In a recent study of organochlorine levels in subjects aged 32 to 85 years in the San Francisco Bay area
organochlorine levels in the control subjects, adjusted for lipid content to account for variation in the lipid
concentration in serum between subjects, were 1030 ng/g lipid for DDE and 229 ng/g lipid for PCBs. Levels in
subjects with pancreatic cancer were higher than in the controls (Hoppin JA, et al. 2000).
The increase in use of PCBs during the 1960s is apparently detectable as increasing concentrations in maternal
sera between 1963 and 1967 (James, R.A. et al. 2002). In the same study, concentrations of certain PCB
congeners, as well as the sum, were significantly higher among nonwhites and increased with calendar date of
blood draw. p,p´-DDT and p,p´-DDE concentrations were about 50% higher for nonwhites compared with
whites and for those born in California or the south-eastern United States versus elsewhere in the United States.
95
Persons who ate Great Lakes sport fish for more than 15 years have two to four times more pollutants in their
serum than non-fisheaters (Schantz SL, et al. 1996). Analysis of serum samples collected from adults (>50 years
old) recreational fishermen in Michigan in 1993-1995 showed significant PCB exposure (mean: 14.26 ppb) with
22 congeners constituting 95% of the total PCBs in most subjects (Humphrey, HEB., et al. 2000). High fish
consumption also correlates with elevated serum CDDs and CDFs (Anderson HA et a1998); the overall mean
concentration of 2,3,7,8-TCCD and TEQs in the serum of recreational fishers from the Great Lakes region were
6.6 ppt and 27.5 respectively. In an unexposed comparison group from Arkansas the serum levels were 2.8 and
15.5 ppt respectively. Levels were statistically different for different lakes (Huron>Michigan>Erie).
In 20 pooled samples of plasma from Inuit blood in northern Quebec the average concentration of 2,3,7,8,-
TCDD was 8.4 ppt (range 2.5 to 81.8) compared to <2 ppt in a comparison group from southern Quebec
(Ayotte P et al 1997).
In a study in the Native American population, results indicated elevated serum PCB levels (mean: 3.7 ppb;
maximum: 9.6 ppb) which correlated with self-reported diabetes and liver disease. Concentrations of hair
mercury were <10 ppm (range: 0.321 ppm9.06 ppm), and serum PCBs were <12 ppb. The average annual fish
consumption rate was 23 grams per day. The investigators have concluded that the Native Americans in their
study tended to be higher consumers of fish, have elevated levels of mercury and PCBs in comparison with the
overall population, and may be at higher risk for health effects (Dellinger JA, et al. 1996).
In the Nurses Health Study (U.S.) plasma concentrations of DDE and PCBs increased in a predictable way with
increasing age and serum cholesterol (Laden, F., et al 1999).
Inuit from northern Quebec had concentrations of hydroxylated polychlorinated biphenyls (OH-PCBs) in whole
blood up to 70 times those from southern Quebec (Sandau, C.D. et al. 2000). An interesting observation in the
same study was that pentachlorophenol was the dominant phenolic compound in blood, with concentrations
found in the northern samples (0.558 to 7.77ng/g on a wet weight basis) generally lower than in the southern
samples (mean 6.29 ng/g).
The median dioxin level in the serum of the Ranch Hand group of Vietnam veterans was 12.2 parts per trillion
(ppt) and the median dioxin level in serum of the control group of veterans was 4.0 ppt (Henriksen G. L. et al
1997).
Gamma-HCH (lindane) is one of the most frequently detected pesticides in the blood of Virginia residents,
highest levels being in the middle age group (Griffith F.D. Jr., and Blanke RV 1975).
Because of the sensitivity of infants to the toxic effects of methylmercury, mercury levels in the blood and hair
(vide inf.) of mothers of childbearing age and children (ages 1-5 years) were measured in the 1999 NHANES
survey. (National Health and Nutrition Examination Survey (NHANES) is a continuous and ongoing survey of
the U.S. civilian population by the Centers for Disease Control and Prevention, Atlanta.) The 90th percentile
values for women aged 16 49 years and for children were 6.2 ppb and 1.4 ppb respectively well below those
considered hazardous (CDCP 2001).
Indians from the English River Wabigoon river system in Canada were exposed to high levels of
methylmercury from eating fish from aquatic system contaminated with mercury. There was evidence of
toxicity among the inhabitants in the mid-1970s when blood concentrations at Grassy Narrows and Whitedog
were 23.80 (range 1.5 to 322.9) ppb and 12.87 (range 1.5 to 172.0) ppb respectively (1976 data). In 1995, the
corresponding concentrations were 7.5 (range 1.7 to 46.7) ppb and 6.1 (range 1.7 to 33.3) ppb respectively (see:
www.ecosuperior).
In both Canada and the U.S., the blood lead level of concern is 10 µg/decilitre. Data from CDC's Third National
Health and Nutrition Examination Survey, Phase 2 (1991--1994) (NHANES) showed that average Blood Lead
Levels (BLLs) in children had decreased approximately 80% since the late 1970s but that elevated BLLs
remained more common among low-income children, urban children, and those living in older housing .Data
obtained in the CDC's Childhood Blood Lead data showed that the proportion of children tested with BLLs >10
µg/dL decreased from 10.5% in 1996 to 7.6% in 1998 in the 19 states providing data . The proportions of
96
children with BLLs >15 µg/dL and >20 µg/dL also decreased. In the U.S. NHANES is an ongoing survey and
average blood lead levels for both children and adults have dropped more than 80 percent since the late 1970's.
There is no comparable regular survey in Canada although there have been numerous targeted blood lead
surveys where the population is considered at high risk. Health Canada estimates that 1 in 20 children have
blood lead levels exceeding 10 µg/decilitre -- a level comparable to that in the U.S.
The dramatic decline in BLLs from the late 1970s through the early 1990s resulted primarily from the phase-out
of leaded gasoline and the resulting decrease in lead emissions, although other exposures also decreased (Pirkle
J.L. ET AL. 1994). A published report on BLLs in children from Vancouver reached similar conclusions (Jin A,
et al. 1995).References to BLLs in several other communities are available, showing generally similar results
(CTFPHC 1994)
3.3.2 Breast milk
Organochlorine compounds tend to selectively partition into adipose tissue and breast milk, which has a higher
fat content than cows' milk. As a result, human breast milk contains these substances in higher concentrations
than in cows' milk.
It has been calculated from 13 studies in Canada and the United States that average DDT levels in breast milk
have steadily declined since 1975 by about one-half in 4.2 5.6 years.
A review of 130 previous studies of global trends in average levels of DDT in breast milk shows a downward
trend in DDT concentrations in breast milk since about 1970. For the U.S. and Canada, the data suggest an 11%
to 21% per year reduction in average levels of DDT in breast milk since 1975 (Smith D. 1999).
In a study of a Native American population living near a Superfund site and exposed to polychlorinated
biphenyls (PCBs) and to a lesser extent, polychlorinated dibenzofurans (PCDFs) and polynuclear polycyclic
aromatic hydrocarbons (PAHs) through consumption of local fish and wildlife, it was shown that there was a
clear correlation between fish consumption and levels of PCBs in breast milk, although this relationship has
decreased as the population has heeded advisories against fish consumption (NIH/NIEHS).
A study of women in Veracruz, Mexico, compared levels of organochlorine pesticides in mothers' fat tissue and
breast milk collected in 1997 and 1998. All were found to contain DDT metabolites or other pesticide
metabolites as well as hexachlorobenzene (HCB) and -hexachlorocyclohexane (-HCH) (Waliszewski S.M., et
al. 1999).
In recent studies, PCB levels in human breast milk ranged from 238 to 271 ng/g lipid weight (Newsome WH, et
al. 1995; Kostyniak PJ, et al. 1999).
No data on levels of toxaphene congeners in breast milk from U.S. women could be located although
documented cases have been reported from Scandinavia and elsewhere.
Dieldrin was detected in 94% of human milk samples taken from in a Canada-wide survey in 1986 (mean: 0.46
ng/g). Comparisons with earlier sampling showed a marked decrease from earlier years (5 ng/g in 1967 and
1970; 2 ng/g in 1975; 1 ng/g in 1982) (Mes J.,. et al. 1986; Newsome, WH., et al. 1995). Levels of DDT and
metabolites in breast milk samples from the Canadian population have shown similar declines. Endrin was
detected in only 1 breast milk sample from an Inuit woman in northern Quebec, and not in samples taken from
Caucasian women living in southern Quebec (Dewailly E. et al 1993).
Heptachlor and heptachlor epoxide have been found in human milk samples from Canadian women (Kutz F. et
al 1977). Levels declined between 1967 and 1982.
3.3.3 Adipose tissue
Residues of DDT and its metabolites declined in adipose tissue samples collected in an annual survey in the U.S.
between 1969 and 1975 (Kutz F. et al 1977).
Concentrations of DDT in adipose tissue indicate human exposure to potential endocrine disrupting substances.
Over 95% of adipose tissue samples taken from the U.S. population contained detectable concentrations of some
97
hormonally active agents, HAA. Although the concentrations were found to be greatest in older individuals,
even children were not immune from exposure.
The most recent U.S. National Human Adipose Tissue survey (NHATS) did not detect endrin in adipose tissues
from the general population (Stanley JS, 1986), neither was endrin detected in cadavers from 6 Canadian
municipalities around the Great Lakes (Williams DT. et al 1988). Endrin was detected in low concentrations in
adipose tissue samples of 3 of 41 residents in British Columbia (Teshke K., et al. 1993). In NHATS, residues of
PCBs were detected in 83% of 46 composite samples in concentrations ranging from 0.014 to 1.7 ppm (lipid
basis), with highest concentrations being from the South Atlantic region (Virginia, North and South Carolina,
Georgia and Florida) (Kutz F. et al. 1977). In 1982, NHATS found -HCH in 87% of 46 composite sample at
levels of <19 to 570 ng/g, highest values being from the southern United States. The estimated national median
level in 1983 was 80 ppb in comparison with a historic level of 140 ppb1 (Mack and Mohadjer 1985). Although
levels of -HCH in adipose tissue continue to decline, the compound continues to be frequently detected.
NHATS detected 2,3,7,8-TCDD in about three-quarters of the samples in the 1982 survey (lipid-adjusted
concentration of 6.2±3.3 ppt). In the 1987 survey, the average concentration for the U.S. population was 5.38
pg/g (±6%) (Orban JE et al 1994).
Analysis of data from 1970 to 1983 revealed that PCB levels in adipose tissue in the U.S. population declined
steadily and that infants, children and youth had lower concentrations of PCBs in their adipose tissue than adults
(Kutz FW et al 1991). Concentrations of three coplanar PCB congeners (77,126,169) were correlated with age
and were higher in males than females (Lordo RA et al 1996).
Levels of heptachlor epoxide in adipose tissues of residents of Louisiana declines for a mean of 239 ppb in 1980
to 159 ppb in 1984 (Holt R.L et al 1986). According to EPA's National Human Monitoring Program and other
broad based U.S. surveys, the geometric mean concentration of oxychlordane in human adipose tissue has
ranged from 90 to 120 ppb between 1971 and 1983 with no clear temporal trend (Adeshina and Todd 1990;
Kutz et al. 1991). North Texas and Canadian studies show increasing oxychlordane levels with age in selected
age groups, but no difference in levels according to sex (Adeshina and Todd 1990; Mes J. 1992).
Toxaphene has not been detected in human adipose tissue in the United States (ATSDR 1996).
3.3.4 Urine
Elevated pentachlorophenol levels in urine and serum have been reported in residents of log homes in Kentucky
and elsewhere (Hosenfeld et al 1986; Cline et al. 1989). In Saskatchewan, Canada pentachlorophenol was
detected in 94% of urine samples at a median concentration of 0.5 ng/ml (Treble and Thompson 1996).
3.3.5 Hair
Scalp hair is a primary indicator to assess exposure to methylmercury. Methylmercury is incorporated into the
hair in proportion to its content in the blood. Once incorporated into the hair strand, methylmercury is stable and
provides a longitudinal history of blood methylmercury levels (IPCS 1990)i. ATSDR has summarized the results
from 10 U.S. communities (ATSDR 1999) but most of the studies were not recent; the range in mean hair
concentrations was 0.473.8 ppm for adults (maximum value of 15.6 ppm) and 0.460.77 ppm for children
(maximum value of 11.3 ppm). The mean concentration of mercury in hair based on a review of existing data
from other countries is 2 µg/g (ppm) (Treble and Thompson 1996).
3.3.6 Miscellaneous
3.3.6.1 IQ
Children of mothers, who ate fish from PCB contaminated water, gave birth to children of low birth weight,
smaller head circumference, hypoactive and abnormal reflexes. At age 11, these children tended to have lower
IQ (6.2 points below average), hyperactivity, and attention deficit disorder. These effects are linked to
prenatal/gestational PCB exposure, not to PCBs in breast milk. Children of mothers, who ate fish from PCB
contaminated water, gave birth to children of low birth weight, smaller head circumference, hypoactive and
abnormal reflexes. At age 11, these children tended to have lower IQ (6.2 points below average), hyperactivity,
98
and attention deficit disorder. These effects are linked to prenatal/gestational PCB exposure, not to PCBs in
breast milk.
3.3.6.2 House dust
House dust is frequently overlooked as a significant source of exposure to pollutants, especially for young
children. In a recent study, samples of dust from a commercial residential cleaning service in North Carolina
revealed the potential for exposure to polycyclic aromatic hydrocarbons and pesticides (pyrethroids), with
increasing concentrations associated with decreasing (i.e., more respirable) particle sizes (Lewis, RG. et al 1999)
3.3.6.3 US/Mexico Border Study
For the past eight years, researchers at the Center for Environmental and Rural Health at Texas A&M University
in collaboration with the Robert Wood Johnson University of Medicine and Dentistry in New Jersey have
conducted environmental monitoring in the Lower Rio Grande Valley region of Texas (Garcia et al. 2001,
Shalat et al. 2002). During this time more than 500 environmental samples including soil, sediment and surface
water have been collected from an area north of El Paso to south of Brownsville. A more focused study has also
been conducted to monitor childhood exposure to pesticides in a rural colonia near Laredo, Texas.
A broad range of organic and inorganic chemicals were detected in environmental media. All of the surface
water samples contained aliphatic hydrocarbons and plasticizers, while soil samples contained these chemicals
as well as pesticides and industrial estrogens. Pesticides were infrequently detected (less than 10% of the
samples) in sediments. The concentration of industrial estrogens, including nonylphenol, octylphenol and
bisphenol, in sediment ranged from below detection to 11 ppm. Industrial estrogens were detected in all of the
Rio Grande River samples collected, with the concentration ranging from 0.01 to 21.12 ppb. In surface soils,
atrazine or atrazine degradation products were detected in over 60% of the samples. Each of the surface soil
samples also contained detectable levels of industrial estrogens.
The pesticide study measured concentrations of organochlorine and organophosphate insecticides in house dust,
hand rinse and urine samples. The concentration of the organophosphate chemicals was generally higher (from
one to two orders of magnitude) than was observed for the organochlorine pesticides, although organochlorines
were detected in house dust more frequently. OPs were detected in 76% of the house dust samples and 50% of
the hand rinse samples. All of the urine samples collected were found to contain at least one pesticide above the
limit of detection. Total OP metabolites in urine ranged from 3.2 to 257 nmol/mol creatinine. Urinary levels
were inversely associated with the age of the child (in months).
3.3.7 Organomercury Compounds
Organic mercury is the most toxic form of mercury. By far the most common organic mercury compound in the
environment is methylmercury. Organic mercury is much more easily absorbed into the body than inorganic
mercury. In pregnant women, it can pass from mother to foetus. Human exposures to high levels of
methylmercury result in neurotoxic effects that have been well documented in a number of incidents globally
(CLS 2000; ATSDR 1999).
Patterns of neurobehavioral damage produced by exposure to methyl mercury during development include
mental retardation, cerebral palsy, deafness and blindness and dysarthria in those exposed in utero, and sensory
system effects, motor or sensorimotor system effects, and cognitive effects in exposed adults.
In humans, there is evidence of effects on the cardiovascular system even at doses below those associated with
neurodevelopmental effects (CLS 2000).
Considerable efforts have been devoted to establishing dose: response relationships for fetal exposure, with
apparently contradictory results. Longitudinal study of child cohorts is continuing (Seychelles, Faroes). Debate
about acceptable exposure involves issues in balancing human nutrition and needs to be resolved in the light of
the dietary importance of fish. Chronic (i.e. life-time) effects of adult exposure to methylmercury are not well
understood, particularly the consequences of repeated but short term (`bolus') exposure characteristic of
subsistence (e.g. aboriginal) and recreational fisheries. Limited guidance is available for dose: response
relationships for adults.
99
From the 1930's to the 1960's, a chemical company dumped tons of mercury into Minamata Bay in Japan.
Thousands of people living around the bay developed methylmercury poisoning through the consumption of
contaminated fish. In one study, excess mortality from cancer of the liver and cancer of the oesophagus was
found in the area with the highest exposure, together with an increased risk for chronic liver disease and
cirrhosis. Consumption of alcoholic beverages was known to be higher than average in the area. In an
examination of the weight of evidence, an IARC Working Group concluded that methylmercury compounds are
possibly carcinogenic to humans (Group 2B) (IARC 1993)
3.3.8 Organotin (Triorganostannic) Compounds
Tributyltin (TBT) is part of a family of organotin compounds in which one to four carbon atoms are bonded to a
tin atom. Tetrabutyltin is a precursor of tributyltin, while dibutyltin and monobutyltin are breakdown products.
Tributyltin is the most toxic of the four compounds in laboratory animals, affecting the immune, endocrine and
central nervous systems. TBT at higher levels is also an extreme irritant to the skin and eye.
A Canadian assessment report concluded: "no quantitative information was identified on the toxicological
effects produced in humans following chronic exposure to non-pesticidal organotin compounds." (Maguire et al.
1993). Available data were considered insufficient to qualitatively or quantitatively estimate exposure of the
general population in Canada to any of the non-pesticidal organostannic compounds.
Methyl-, Octyl- and Dodecyltin stabilizers have had widespread national approvals for food-contact applications
over many years. A recent assessment within the EU concluded that the use of these organostannic stabilizers in
PVC for food packaging is not a risk for the consumer (Summer K. H. et al 1996).
Levels of tin have been reported in human adipose tissue samples during the 1982 National Human Adipose
Tissue Survey at concentrations ranging from 8.7 to 15 µg/g (Stanley 1986).
3.3.9 Organolead Compounds
Short-term exposure to high levels of lead can cause a metallic taste, abdominal pain, vomiting, diarrhoea,
convulsions, coma or even death. However, very few cases of acute lead poisoning occur in North America
except through occupational exposure.
Although organic lead compounds were formerly used as a gasoline additive, these compounds are largely
destroyed in the combustion process or by atmospheric oxidation to inorganic lead (ATSDR 1999).
While the most evident manifestation of chronic exposure is anaemia, exposure has also been linked to impaired
mental function, visual-motor performance and neurological damage in children, and memory and attention span
(FPCEOH 1994; ATSDR 1999). Exposure is also associated with lack of appetite, abdominal pain and
constipation, fatigue, sleeplessness, irritability and headache. Continued excessive exposure to lead (such as
may occur in an industrial setting) may affect kidney function.
Children are particularly at risk. They absorb lead more easily than adults do. Children also absorb a higher
proportion of lead from all sources (air, food, water and dust for example) than adults. Contaminated dust is a
particularly important source of exposure for babies and young children. During pregnancy, lead can cross the
placenta and reach the tissue of the unborn child. The last trimester of pregnancy may be the most critical time
for this to occur. In the past, increased spontaneous abortions and stillbirth rates were noted in female industrial
workers exposed to high levels of lead.
Over the past decade, some researchers have found that exposure to even low levels of lead prior to birth, or
during infancy and early childhood, may cause impairment to intellectual development, behavioural
disturbances, decreased childhood size and hearing impairment.
3.4 Data Gaps and Programme Needs
3.4.1 Ecological Data Gaps
The study by Muir et al. (2001) identified a number of specific data gaps but noted as well, that there is an
overall lack of data on PTSs in North America. Of the 100 chemicals in the Canadian Government list of
chemicals (Environment Canada 2002b) , which meet the criteria for persistence, toxicity, long-range transport
100
and bioaccumulation, with the exception of two or three pesticides, there is very limited information available
on the physical properties, half-lives of persistence, bioaccumulation, or current environmental levels of these
compounds. While there are environmental measurement data on some of the 100, the vast majority of the
compounds remain unstudied (Muir et al. 2001).
Basic physical property information is needed on "new" chemicals, in order to predict environmental fate and
bioaccumulation potential. Currently, Kow values are only available based on structure activity. Biodegradation,
photolysis and hydrolysis data, which are needed to predict overall environmental persistence and LRTAP
potential, are lacking for most new PTSs (Muir et al. 2001).
Basic toxicology information (e.g. LD50 estimates) for new PTSs is needed on the mammalian, avian and
aquatic species (Muir et al. 2001).
In the mountainous sub-region bounding the western edge of the North American region and including most of
Mexico, atmospheric, terrestrial and freshwater compartments and pathways should be evaluated in a
comprehensive and systematic way to determine the levels and consequences of PTSs in this area, the extent of
focusing due to the elevation and the relative importance of long-range offshore air pathways.
3.4.2 Specific data gaps include:
· PTSs levels for species in all environmental compartments, especially aquatic and terrestrial.
· PAH levels in biota as epoxides and hydroxylated derivatives.
· The toxicology of individual compounds e.g., Toxaphene.
· The toxicology, fate, transport and bioaccumulation potential of PFOS.
· Levels in aquatic biota and elsewhere of new chemicals, including: PBDEs, TBBPA, Chlorinated Paraffins,
Polychlorinated Naphthalenes (PCNs) and other dioxin-like compounds (e.g. dioxin-like PCBs).
· The identification and study of new potentially persistent chemicals such as dacthal, flutriafol and
perfluorocarboxylates.
· Anthropogenic versus natural sources of mercury, and their relative biological availability.
· The extent of conversion of various forms of mercury, its reverse cycle to elemental mercury and its transfer
from the atmosphere to aquatic food webs.
3.4.3 Programme Needs
Tri-national research and monitoring programs on PTSs with a continental focus are needed to ensure that
information on current levels and time trends of old and emerging PTSs are available and are shared in all
regions in major environmental media, e.g., water, air, sediments and fish (Muir et al. 2001) and in the area of
human health. In some cases it is not the data alone that are missing but also the ability/capacity to gather
appropriate data.
In order to produce reliable data which are accepted by the scientific community as a whole and to allow inter-
comparisons and the confirmation of results from participating laboratories, efforts should be made to establish
uniformity of analytical methodologies.
The capacity to link the chemical measurements to biological effects needs to be strengthened through long-term
field studies and support for training (Muir et al. 2001).
Modeling capability for forecasting the trends in environmental levels and fate of old and new PTSs needs to be
refined and new approaches for modeling need to be explored. (Muir et al. 2001).
Analytical methods information is needed for new PTSs.
Method development is limited by lack of facilities such as clean rooms to limit contamination from products in
current use as well as lack of appropriate instrumentation (Muir et al. 2001).
Commitments to long-term research and monitoring programs are needed to assessments of time trends in PTSs
resulting from UN ECE and UNEP agreements. In the Great Lakes Basin the situation is not satisfactory; in
other sub regions it is worse. For example, in North America, there are no long-term trends measurements for
PTSs in surface waters (Muir et al. 2001).
101
3.5 Conclusions
Because of the absence of information on the levels and trends of PTSs in Mexico, the risk posed by these
compounds to the environment and to human health, particularly those associated with drinking water cannot be
assessed.
For most of North America, there is little information on the environmental levels, trends and consequences of
PTSs. The high quality and extensive coverage of research in the Great Lakes Basin stands in sharp contrast
with the rest of the region.
The Great lakes
Three decades ago elevated levels of a wide range of PTSs were associated with an array of impacts on wild life
and risk to human health. Since the 1970's there have been significant recoveries in reproductive success and
increases in population for most of the affected, fish-eating, bird species.
Although natural reproduction now occurs in Lake Superior and stocking has been discontinued, natural
reproduction in the other Great Lakes is very low or non-existent and lake trout populations are solely
maintained by stocking.
In 1991, concentrations of toxic chemicals in the open waters of the Great Lakes are well below Canadian and
international drinking water standards.
Though the temporal trend in annual flow of pesticide into the five Great Lakes is generally decreasing, the
presence of toxic substances in the Great Lakes basin continues to be a significant concern for both wild life and
human health. Contaminant-related restrictions on the harvest of many commercial fish species are still in place
along with fish consumption advisories.
Downward fluxes for in-use pesticides, such as -HCH, and PAHs account for the highest fluxes into the lakes.
The exchange fluxes for all PAHs show no real temporal trend.
In the Great Lakes, volatilization (upward) fluxes for banned pesticides are almost 10 times greater than those
for in-use pesticides. The trend in gas exchange of PCBs, Dieldrin and p,p'-DDE has been in the direction of net
volatilization indicating that the lakes are acting as sources.
The Region
Despite the declining levels, the interim guideline for PCB of 0.32 pg TEQ/g ww, designed to protect Canadian
wildlife that consume fish and shellfish, is routinely exceeded by both predator and forage fish in many areas.
Levels of PCBs and DDT have declined significantly in top predators. For example, continued decline in PCBs
in herring gull and osprey eggs reflects lower emissions following controls on open uses. Declines in Great
Lakes lake trout and walleye have not been as dramatic especially since the mid-1980s reflecting continued
emissions from urban areas and recycling of contaminants within the lakes.
Levels of mercury, unlike the PCBs and DDT, have increased in the past 20 years in fish-eating birds and
mammals, particularly near urban centres. The interim guideline of Hg of 22 ng/g ww for protection of fish
consuming wildlife is exceeded in almost all fish measured to date in Canada. However, average mercury
concentrations in insectivorous fish such as lake whitefish and white sucker are well below this guideline.
From sediment studies it has been shown that urban areas are an important sources of many of PTSs e.g. PCBs,
chlorobenzenes and PBDEs (such as BDE209).
Current use OCs, endosulfan and lindane and the high volume herbicides atrazine are reaching remote lakes.
Recent observations suggest that concentrations as low as 100 ng/L affect ecologically important aquatic
systems. The remote lakes could have levels approaching that threshold.
A lake water and sediment survey (1998 2001) across the northern part of the region, included many new
potentially persistent chemicals not previously identified in remote lake surface waters, such as dacthal, the
fungicides flutriafol and chlorothalonil, deca-bromobiphenyl ether (a PBDE) and the PFOS, perfluorooactanoic
acid, a product recently removed from the market by its manufacturer, 3M Corp.
102
Recent studies of PCB, CDDs and CDFs have shown that persons who ate Great Lakes sport fish for more than
15 years have two to four times higher levels in their serum than non-fisheaters .
A 1999 review of 130 previous studies of global trends in average levels of DDT in breast milk, show a
downward trend in DDT concentrations in breast milk since about 1970. For the U.S. and Canada, the data
suggest an 11% to 21% per year reduction in average levels of DDT in breast milk since 1975.
Dieldrin, DDT and metabolites in breast milk samples from the Canadian population have shown declines over
the past three decades.
Growing evidence of the occurrence and potential impacts of new chemicals suggest that detailed assessments
and timely reviews should be conducted on flame retardants (PBDEs, PBBS and TBBPA), short chain
chlorinated paraffins, perfluorooctane sulfonate (PFOS), Polychlorinated naphthalenes (PCNs), Dioxin-like
PCBs and alkylphenol ethoxylates (APEs).
Detailed studies of environmental concentration and associated observed effects are rare. In the case or
emerging PTSs, basic ecotoxicological data is often not available.
3.6 References
Addison, R.F. and T.G. Smith. 1998. Trends in organochlorine residue concentrations in ringed seal (Phoca
hispida) from Holman NWT, 1972-1991. Arctic 51: 253-261.
Alaee M, Wenning R.J. 2002. Understanding the significance of brominated flame retardants in the
environment. Current understanding, issues and challenges. Chemosphere, 46 (5) 579-582.
AMAP 1998. AMAP Assessment Report. Arctic Monitoring and Assessment Programme secretariat. Norway.
ATSDR 1999. Toxicological Profile for Mercury, Agency for Toxic Substances and Disease Registers. Atlanta,
GA.
Bailey, G.E., D.E. Goeger and J. Hendricks, 1989. Factors influencing experimental carcinogenesis in laboratory
fish models. In: U. Varanasi (ed.). Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment,
pp. 253-268. CRC Press, Boca Raton, Florida.
Bergman, A., M. Olsson and S. Reiland, 1992a. Skull-bone lesions in the Baltic grey seal (Halichoerus grypus).
Ambio 21: 517-519.
Bergman, Å., M. Athanasiadou, S. Bergek, K. Haraguchi, S. Jensen and E.Klasson Wehler, 1992b. PCB and
PCB methyl sulphones in mink treated with PCB and various PCB fractions. Ambio 21: 570-576.
Bergman, Å., E. Klasson Wehler and H. Kuroki, 1994a. Selective retention of hydroxylated metabolites in
blood. Environ. Hlth Persp. 102: 464-469.
Bergman Å., R.J. Norstrom, K. Haraguchi, H. Kuroki and P. Béland, 1994b. PCB and DDE methyl sulphones in
mammals from Canada and Sweden. Environ. Toxicol. Chem. 13: 121-128.
Bernhoft, A., H. Hektoen, J.U. Skaare and K. Ingebrigtsen, 1994. Tissue distribution and effects on hepatic
xenobiotic metabolising enzymes of 2,3,3',4,4'-pentachlorobiphenyl (PCB-105) in cod (Gadus morhua) and
rainbow trout (Oncorhynchus mykiss). Environ. Pollut. 85: 351-359.
Bernhoft, A., Ø. Wiig and J.U. Skaare, 1997. Organochlorines in polar bears (Ursus maritimus) at Svalbard.
Environ. Pollut. 95(2): 159-175.
Boon, J.P., E. van Arnhem, S. Jansen, N. Kannan, G. Petrick, D. Schulz, J.C. Duinker, P.J.H. Reijnders and A.
Goksøyr, 1992. The toxicokinetics of PCBs in marine mammals with special reference to possible interactions
of individual congeners with the cytochrome P450-depen-dent monooxygenase system: an overview. In: C.H.
Walker and D.R. Livingstone (eds). Persistent pollutants in marine ecosystems, pp. 119-159. Pergamon Press,
Oxford.
Botello A. V., L. Rueda-Quintana, G. Díaz-González and A. Toledo 2000. Persistent Organochlorine Pesticides
(POPS) in Coastal Lagoons of the Subtropical Mexican Pacific, Bull. Environ. Contam. Toxicol. 64:390-397.
103
Brandt, I., C-J. Jönsson and B-O. Lund, 1992. Comparative studies on adrenocorticolytic DDT-metabolites.
Ambio 21: 602-605.
Braune, B.M., G.M. Donaldson and K.A. Hobson 2001. Contaminant residues in seabird eggs from the
Canadian Artic. Part I. Temporal trends 1975-1998. Environ. Pollut. 114: 39-54.
Brouwer, A., W.S. Blaner, A. Kukler and K.J. van den Berg, 1988. Study on the mechanism of interference
of 3,4,3',4'-tetrachlorobiphenyl with the plasma retinol-binding proteins in rodents. Chem.-Biol. Interact. 68:
203-217.
Brouwer, A., E. Klasson-Wehler, M. Bokdam, D.C. Morse and W.A. Traag, 1990. Competitive inhibition of
thyroxin binding to trans-thyretin by monohydroxy metabolites of 3,4,3',4'-tetrachlorobi-phenyl. Chemosphere
20: 1257-1262.
BSEF. 2000. Bromine Science and Environmental Forum. http://www.ebfrip.org/download/weeeqa.pdf
Clarkson, T.W., 1986. Effects - general principles underlying the toxic action of metals. In: L. Friberg, G.F.
Nordberg and V.B. Vouk (eds.). Handbook on the toxicology of metals, Vol. I, pp. 128-148. Elsevier Science
Publishers, Amsterdam.
CLS 2000. Toxicological effects of methyl mercury, Commission on Life Sciences, National Research Council,
National Academy of Sciences, National Academy Press, Washington D.C.
Czuczwa, J.M. and R.A. Hites, 1984. Environmental fate of combustion-generated polychlorinated dioxins and
furans. Environ. Sci Technol.18: 444-450.
Debets, F.M.H. and J.J.T.W.A. Strik, 1979. An approach to elucidate the mechanism of hexachlorobenzene-
induced hepatic porphyria, as a model for the hepatotoxicity of polyhalogenated aromatic compounds (PHA's).
In: J.J.T.W.A. Strik and J.H. Koemans (eds). Chemical por-phyria in man, pp. 181-208. Elsevier/North Holland
Biomedical Press, Amsterdam.
Environment Canada 1995. Toxic Substances Management Policy. Environment Canada, Ottawa.
Environment Canada 1998. Interim Canadian tissue residue guideline for methyl mercury. Ecosystems Science
Directorate, Ottawa, ON.
Environment Canada 2000a. Great Lakes Fact Sheet Contaminants in Herring Gull Eggs from the Great
Lakes: 25 Years of Monitoring Levels and Effects.
Environment Canada 2000b. Great Lakes Fact Sheet The Rise of the Double-crested Cormorant on the Great
Lakes: Winning the War Against Contaminants.
Environment Canada 2000c. Great Lakes Fact Sheet The Fall and Rise of Osprey Populations in the Great
Lakes Basin.
Environment Canada, 2001. Great Lakes Fact Sheet Bald Eagle Populations in the Great Lakes Region.
Prepared in collaboration with the Ontario Ministry of Natural Resources and Bird Studies Canada.
Environment Canada 2002a. High-Altitude POPs and Alpine Predators, Science and Environments Bullitin,
Environment Canada http://www.ec.gc.ca/science/sandenov00/article1_e.html
Environment Canada 2002b. Priority Substances Assessment Program, Canada. http://www.hc-sc.gc.ca/hecs-
sesc/exsd/psap.htm
Espeland, O., L. Kleivane, S. Haugen and J.U. Skaare, 1997. Organochlorines in mother and pup pairs in two
arctic seal species: Harp seal (Phoca groenlandica) and hooded seal (Cystophora crista-ta). Mar. Environ. Res.
44(3): 315-330.
Evers, D.C., J.D. Kaplan, M.W. Meyer, P.S. Reaman, W.E. Braselton, A. Major, N. Burgess and A.M.
Scheuhammer 1998. Geographic trend in mercury measured in common loon feathers and blood. Environ.
Toxicol. Chem. 17: 173-183.
104
Farrington, J.W., Goldberg, E.D., Risebrough, R.W., Martin, J.H., Bowen, V.T. 1983. US ``Mussel Watch''
19761978: an overview of the trace metal, DDE, PCB, hydrocarbon, and arti.cial radionuclide data.
Environmental Science and Technology 17, 490-496.
Foulkes, E. C. 1988. On the mechanism of transfer of heavy metals across cell membranes, Toxicology , 52:
263-272.
Garcia, SS, C Ake, B Clement, HJ Huebner, KC Donnelly and SL Shalat 2001. Initial results of environmental
monitoring in the Texas Rio Grande Valley. Environ. International 26:465-474.
Giesy, J. P. and K. Kannan. 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environmental
Science and Technology 35: 1339-1342.
Government of Canada 1991. Toxic Chemicals in the Great Lakes and Associated Effects Synopsis,
Departments of Environment, Fisheries and Oceans and Health and Welfare, Ottawa.
Grasman, K.A., C.A. Bishop, W.W. Bowerman, J.P. Ludwig, P.A. Martin, L. Lambert 2002. Lake Erie LaMP
Beneficial Use Assessment: Animal Deformities and Reproduction Impairment. Technical Report Series No.
362. Canadian Wildlife Service, Ontario Region, Burlington, Ontario, Canada.
Great Lakes Binational 2000. Progress Report 2000, Great Lakes Binational Toxics Strategy.
http://www.binational.net/bns/progress/index.html
Great Lakes Binational 2001. State of the Great Lakes - 2001 Report, Great Lakes Binational Toxics Strategy,
http://www.binational.net/sogl2001/download.html
Great Lakes Commission 2002. Human Health and the Great Lakes. Ann Arbor, USA
http://www.great-lakes.net/humanhealth/other/pops.html
Gschwend, P.M. and R.A. Hites. 1981. Fluxes of polycyclic aromatic hydrocarbon into marine and lacustrine
sediments in the northeastern United State. Geochim. Cosmochem. Acta 45: 2359-2367.
Gubala, C.P., D.H. Landers, M. Monetti, M. Heit, T. Wade, B. Lasorsa, and S. Allen-Gil. 1995. The rates of
accumulation and chronologies of atmospherically derived pollutants in Arctic Alaska, USA. Sci. Total Environ.
160/161, 347 - 362.
Hansen, K., L. A. Clemen, M. E. Ellefson and H. O. Johnson. 2001. Compound-specific, quantitative
characterization of organic fluorochemicals in biological matrices. Environmental Science and Technology 35:
766-770.
Haraguchi, K., Å. Bergman, M. Athanasiadou, E. Jakobsson, M. Olsson and Y. Masuda, 1990. PCB methyl
sulphones in grey seal and otter from Swedish environment. Organohalogen Compounds 1: 415-418.
Haraguchi, K., M. Athanasiadou, Å. Bergman, L. Hovander and S. Jen-sen, 1992. PCB and PCB methyl
sulphones in selected groups of seals from Swedish waters. Ambio 21: 546-549.
Hayes, T.B., A. Collins, M. Lee, M. Mendoza, N. Noriega, A. A. Stuart and Aaron Vonk 2002. Hermaphroditic,
demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl.
Acad. Sci., 99 (8), 5476-5480.
Henriksen, E.O., G.W. Gabrielsen and J.U. Skaare, 1996. Levels and congener pattern of polychlorinated
biphenyls in kittiwakes (Rissa tri-dactyla), in relation to mobilization of body-lipids associated with
reproduction. Environ. Pollut. 92: 27-37.
Heit, M., Y. Tan, C. Klusek and J.C. Burke. 1981. Anthropogenic trace elements and polycyclic aromatic
hydrocarbon levels in sediment cores from two lakes in the Adirondack acid lake region. Water, Air and Soil
Pollut. 15: 441-464.
Hites, R.A., R.E. Laflamme and J.W. Farrington. 1977. Polycyclic aromatic hydrocarbons in recent sediments:
the historical record. Science 198:829-831.
105
Hites, R. A. and R. James. 1999. Chlorothalonil and Dacthal in Great Lakes air and precipitation samples.
Journal of Great Lakes Research 25(2): 406-411.
Hoff, R.M., W.J.M. Strachan, C.W. Sweet, C.H. Chan, M. Shackleton, T.F. Bidleman, K.A. Brice, D.A.
Burniston, S. Cussion, D.F. Gatz, K. Harlin and W.H. Schroeder. 1996. Atmospheric deposition of toxic
chemicals to the Great Lakes: A review of data through 1994. Atmos. Environ. 30, 3505-3527.
Högberg, J. and J. Alexander, 1986. Selenium. In: L. Friberg, G.F. Nord-berg, and V.B. Vouk (eds.). Handbook
on the toxicology of metals, Vol. II, pp. 482-520. Elsevier Science Publishers, Amsterdam.
Hooper, K, and TA McDonald 2000. The PBDEs: an emerging environmental challenge and another reason for
breast-milk monitoring programs. Environmental Health Perspectives 108(May): 387.
Hu, W. Y., P. D. Jones, B. L. Upham, J. P. Geisy and J. E. Trosko. 2001. SETAC Europe, 11th Annual Meeting,
Madrid, Spain.
IADN, 1998a. Technical Summary of Progress under the Integrated Atmospheric Deposition Program 1990-
1996, pp 107.
IADN, 1998b. Atmospheric Deposition of Toxic Substances to the Great Lakes: IADN Results through 1998.
Environment Canada and the United States Environmental Protection Agency, pp 88.
IARC 1993. Monographs on the Evaluation of Carcinogenic Risks to Humans. Mercury and mercury
compounds, Vol.58.
IJC 1978. Great Lakes Water Quality Agreement of 1978, International Joint Commission United States and
Canada. http://www.ijc.org/agree/quality.html
IJC 1987. Great Lakes Water Quality Agreement as amended 1987, International Joint Commission United
States and Canada. http://www.ijc.org/ijcweb-e.html
Ikonomou, M, Rayne, S, Addison, R. 2002. Exponential increases of brominated flame retardants,
polybrominated diphenyl ethers in the Canadian Arctic from 1981 to 2000. Environ. Sci. Technol. 2002, 36,
1886-1892.
IPCS 1990. International Programme On Chemical Safety, Methylmercury, Environmental Health Criteria 101
James, R.A., Hertz-Picciotti, I., Willmen, E., Keller, JA.,Charles M.J. 2002. Determination of Serum
Polychlorinated Biphenyls and Organochlorine Pesticides measured in Women from the Child Health and
Development Study Cohort, 1963-67
Jansson, B., S. Jensen, M. Olsson, L. Renberg, G. Sundström and R. Vaz, 1975. Identification by GC-MS of
phenolic metabolites of PCB and p,p'-DDE isolated from Baltic guillemot and seal. Ambio 4: 87-92.
Jensen, S., A.G. Johnels, M. Olsson and G. Otterlind, 1969. DDT and PCBs in marine animals from Swedish
waters. Nature 224: 247-250.
Kannan, K., et al. 2001. Accumulation of perfluorooctane sulfonate in marine mammals. Environmental Science
and Technology 35: 1593-1598.
Kannan, K., N. Yamashita, T. Imagawa, W. Decoen, J. S. Khim, R. M. Day, C. L. Summer and J. P. Giesy.
2000. Polychlorinated naphthlalenes and polychlorinated biphenyls in fishes from Michigan waters including
the Great Lakes. Environmental Science and Technology 35(4): 566-572.
Kester, M.H.A., S. Bulduk, D. Tibboel, W. Meinl, H. Glatt, C.N. Falany, M.W.H. Coughtrie, A. Bergman, S.H.
Safe, G.G.J.M. Kuiper, A.G. Schuur, A. Brouwer and T.J. Visser. 2000. Potent inhibition of estrogen
sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs.
Endocrinology 141(5): 1897-1900.
Kihlström, J.E., M. Olsson, S. Jensen, Å Johansson, J. Ahlbom and Å. Bergman, 1992. Effects of PCB and
different fractions of PCB on the reproduction of the mink (Mustela vison). Ambio 21: 563-569.
106
Kolic T.M., K.A. MacPherson, E.J. Reiner, T.Gobran and A.Hayton 2000. A Comparison Of TEQ Contributions
From Chlorinated Dioxins, Furans And Dioxin-Like PCBs In Great Lakes Fish, Organohalogen Compounds,
46: 562,
Kutz F.,Yobs A., Strassman S. et al 1977. Pesticides in people: Effects of reducing DDT usage on total DDT
storage in humans. Pesticide Monitoring J. 11(2): 61 63.
Larsen, J.E., M. Celander and A. Goksøyr, 1992. The cytochrome P450 system of Atlantic salmon (Salmo salar)
II. Variations in hepatic catalytic activities and isozyme pattern during an annual reproductive cycle. Fish
Physiol. Biochem. 10: 291-301.
Lebedev, A. T., Poliakova, O. V., Karakhanova, N. K., Petrosyan, V. S., Renzoni A. (1998): The
contamination of birds with organic pollutants in the Lake Baikal region. Sci. Tot. Environ., 212, 153-162.
Letcher, R.J., R.J. Norstrom and Å. Bergman, 1995a. Geographical distribution and identification of methyl
sulphone PCB and DDE metabo-lites in pooled polar bear (Ursus maritimus) adipose tissue from western
hemisphere Arctic and subarctic regions. Sci. Total Environ. 160/161: 409-420.
Letcher, R.J., R.J. Norstrom and Å. Bergman, 1995b. An integrated analytical method for determination of
polychlorinated aryl methyl sulfone metabolites and polychlorinated hydrocarbon contaminants in biological
matrices. Anal. Chem. 67: 4155-4163.
LMLMP 2002. Lake Michigan Mass Balance Study, reported in Lake Michigan LaMP Highlights 2002
http://www.epa.gov/grtlakes/lakemich/lm02/index.html.
Lockhart, W.L., P. Wilkinson, B. N. Billeck, R.V. Hunt, R. Wagemann, and G. Brunskill. 1995. Current and
historical inputs of mercury to high-latitude lakes in Canada and to Hudson Bay. Water, Soil, Air Pollut. 80:
603-610.
Lockhart, W.L., P. Wilkinson, B. N. Billeck, R.A. Danell, R.V. Hunt, J. Delaronde and V. St. Louis. 1998.
Fluxes of mercury to lake sediments in central and northern Canada inferred from dated lake sediment cores.
Biogeochemistry 40:163-173.
Lund, B.O., Å. Bergman and I. Brandt, 1988. Metabolic activation and toxicity of a DDT-metabolite, 3-
methylsulphonyl-DDE, in the adrenal zona fasciculata in mice. Tem. Biol. Interactions 65: 25-40.
Luross, J. M., C. M. Cannon, M. Alaee, D. B. Sergeant, D. M. Whittle and K. R. Solomon. 2001. Spatial
distribution and temporal trends in PBBs and PBDEs in lake trout from the Great Lakes. International
Association for Great Lakes Research 44th Conference, Green Bay, WI.
Maguire R.J., Long G., Meek M.E., Savard S. (1993) Canadian Environmental Protection Act Priority
Substances List Assessment Report Non-Pesticidal Organotin Compounds. Health Canada, Ottawa, ON., K1A
0L2
Manchester-Neesvig, J. B., K. Valters and W. C. Sonzogni. 2001. Comparison of polybrominated diphenyl
ethers (PBDEs) and polychlorinated biphenyls (PCBs) in Lake Michigan salmonids. Environmental Science and
Technology 35(6): 1072-1077.
Martin, M., Gutierrez-Galindo, E. 1989. Pesticides and PCBs in oysters from Mazatl an, Sinaloa, Mexico.
Marine Pollution Bulletin, 20, 469472.
Marvin C., Mehran A., S. Painter, M. Charlton, P. Kauss, T. Kolic, K. MacPherson, D. Takeuchi and E. Reiner
2002. Persistent Oorganic Pollutants in Detroit River Suspended Sediments: Polychlorinated Dibenzo-p-dioxins
and Dibenzofurans, Dioxin-like Polychlorinated Biphenyl's and Ppolychlorinated Naphthalene's, Chemosphere
49, 111120.
Marvin C., Gary Stern, Eric Reiner, Karen MacPherson, Terry Kolic, Eric Braekevelt and Scott Painter 2002.
Environmental Levels and Trends - Spatial and Temporal Trends in Persistent Organic Pollutants in lake
Ontario Sediments Organohalogen Compounds 56: 453
107
Meironyte, D., K. Noren and A. Bergman. 1999. Journal of Toxicology and Environmental Health A 58: 329-
341.
Muir, D.C.G., A. Omelchenko, N. P. Grift, D.A. Savoie, W. L. Lockhart, P. Wilkinson, and G. J. Brunskill.
1996. Spatial Trends and Historical Deposition of Polychlorinated Biphenyls in Canadian Mid-latitude and
Arctic Lake Sediments. Environ. Sci Technol. 30: 3609-3617.
Muir, D.C.G., Stern, G.A. and Karlsson, H. (1999) Organohalogen Compounds. 41: 565.
Muir, D., M. Alaee, D. Dube, L. Lockhart and T. Bidleman, 2001. Chapter 4, Persistent Organic Pollutants and
Mercury. In: Environment Canada. Threats to Sources of Drinking Water and Aquatic Ecosystem Health in
Canada, pp. 13-16.
Muir, D., R. Anderson, K. Drouillard, M. Evans, W. Fairchild, S. Guildford, D. Haffner, K. Kidd, J. Payne, M.
Whittle 2002a. Biomagnification Of Persistent Organic Pollutants And Mercury In Canadian Freshwater
Subsistence Fisheries And Food Webs. Toxic Substances Research Initiative Project #236 Final Report,
National Water Research Institute, Environment Canada.
Muir D., M. Douglas, R. Pienitz, W. Vincent, F. Wania 2002b. Sources, Long Range Transport and Impacts of
New and Old POPs Inferred from Dated Lake Sediment Cores and Lake Waters. Toxic Substances Research
Initiative Project #206 Final Report, National Water Research Institute, Environment Canada.
Murphy, T.J. and C.P. Rzeszutko 1977. Precipitation inputs of PCBs to Lake Michigan. J. Great Lakes Res. 3,
305-312.
Nebert, D.W. and F.J. Gonzalez, 1987. P450 genes: Structure, evolution, and regulation. Ann. Rev. Biochem. 56:
945-993.
NGS 2002. Downside of Tourism - Mountain Ecosystems in Danger Worldwide, National Geographic Society.
http://news.nationalgeographic.com/news/2002/02/0201_020201_wiremountain.html
Noren, K. and Mieronyte D. 1998. Contaminants in Swedish human milk. Decreasing levels of organochlorine
and increasing levels of organobromine compounds. Organohalogen Compounds 35:1-4.
OMOE 2001. Guide to Eating Ontario Sport Fish 2001-2002, Ontario Ministry of the Environment, Queen's
Printer for Ontario, Toronto, Ontario. http://www.ene.gov.on.ca/envision/guide/
Paez-Osuna, F., Osuna-Lopez, J.I., Vazquez-Botello, A.V. 1998. Biomonitoreo de la contaminaci.n en las aguas
costeras del Pacifio subtropical Mexicano: metales pesados, plaguicidas a hidrocarburos del petroleo. Informe
Tecnico Academico Final, Proyecto Conacyt 0185PT, M exico.
PANNA 2002a. Pesticide Action Network North America (PANNA): Pesticide Database. San Francisco.
http://www.pesticideinfo.org/
PANNA 2002b. Pesticide Action Network North America (PANNA): Endocrine disruptors. San Francisco.
http://docs.pesticideinfo.org/documentation3/EndocrineDisruptors
Pearson, R.F., D.L. Swackhamer, S.J.Eisenreich, and D.T. Long. 1997a. Assessing the importance of
atmospheric deposition of PCDD/Fs to the Great Lakes.Compositional comparons of PCDD/F sedimentary
accumulations. Organohalogen Compounds 33, 76-81.
Pearson, R.F., D.L. Swackhamer, S.J.Eisenreich, and D.T. Long. 1997b. Concentrations, accumulations, and
inventories of toxaphene in sediments of the Great Lakes. Environ. Sci. Technol. 31, 3523-3529.
Pirkle JL, Brody DJ, Gunter EW, et al. 1994. The decline in BLLs in the United States: the National Health and
Nutrition Examination Surveys. JAMA. 272:284--91.
Pierce, R.C., D.M. Whittle and J.B. Bramwell (ed.) 1998. Chemical contaminants in Canadian aquatic
ecosystems. Department of Fisheries and Oceans. ISBN 0-660-17475-8. 329 p.
Polischuk, S.C., R.J. Letcher, R.J. Norstrom and M.A. Ramsay, 1995. Preliminary results on the kinetics of
organochlorines in Western Hudson Bay polar bear (Ursus maritimus) Sci. Total Environ. 160/161: 465-472.
108
Poliakova O.V, Lebedev A.T., Petrosyan V.S., Hanninen O., Renzoni A., Savva D. and Walker C. 2000.
Accumulation of persistent organic pollutants in the food chain of lake Baikal, Toxicological And
Environmental Chemistry, 75, 235-243.
Polischuk, S.C., R.J. Letcher, R.J. Norstrom and M.A. Ramsay, 1995. Preli-minary results on the kinetics of
organochlorines in Western Hudson Bay polar bear (Ursus maritimus) Sci. Total Environ. 160/161: 465-472.
Renner, G., 1988. Hexachlorobenzene and its metabolism. Toxicol. En-viron. Chem. 18: 51-78.
Rosales-Hoz M. L, Escalona R. L. 1983. Organochlorine residues in organisms of two coastal lagoons of
Northwest Mexico, Bull Environ Contam Toxicol, 30: 456-463.
Rosales-Hoz M. L, Escalona R. L, Alarcon R. M, Zamora V 1985. Organochlorine hydrocarbons residues in
sediments of two coastal lagoons of Northwest Mexico, Bull Environ Contam Toxicol, 35: 322- 330.
Sanderson, M. and R. Frank 1977. Cited in Background to the Regulation of Polychlorinated Biphenyls (PCB)
in Canada, Report of the PCB Task Force 76-1, p.169.
Sericano, J.L., Wade, T.L., Jackson, T.J., Brooks, J.M., Tripp, B.W., Farrington, J.W., Mee, L.D., Readman,
J.W., Villeneuve, J.P., Goldberg, E.D. 1995. Trace organic contamination in the Americas: an overview of the
US National Status & Trends and the International Mussel Watch programmes. Marine Pollution Bulletin 31,
214225.
Shalat, SL, KC Donnelly, NCG Freeman, JA Calvin, S Ramesh, M Jimenez, K Black, C Couthino, L Needham,
D Barr and J Ramirez 2002. Nondietary ingestion of pesticides by children in an agricultural community on the
US/Mexico border: Preliminary results. Journ. Exposure Analysis and Environ. Epidemiology 12: (In press).
Smith, R.M., P.W. O'Keefe, D.R. Hiker, B. Bush, S. Connor, R. Donnelly, R. Storm and M. Liddle. 1993. The
historical record of PCDDs, PCDFs, PAHs, PCBs and lead in Green Lake, New York 1860-1990.
Organohalogen Compounds 12, 215-218.
Stanley JS. 1986. Broad scan analysis of the FY82 national human adipose tissue survey specimens. Vol. I.
Executive summary. Report to U.S. Environmental Protection Agency, Office of Toxic Substances, Washington,
DC, by Midwest Research Institute, Kansas City, MO. EPA-566/5-86-035.
Stein, J.E., T. Hom and U. Varanasi, 1984. Simultaneous exposure of English sole (Parophrys vetulus) to
sediment-associated xenobiotics: I. Uptake and disposition of [14C]-polyhlorinated biphenyls and [3H]-benzo[
a]pyrene. Mar. Environ. Res. 13: 97-119.
Stern, G. and R. Addison. 1999. Temporal trends of organochlorines in southeast Baffin beluga and Holman
ringed seal, p. 203-212. In S. Kalhok (ed.), Synopsis of research conducted under the 1998/99 Northern
Contaminants Program, Ottawa. Indian and Northern Affairs Canada.
Strachan, W.M.J. and H. Huneault 1979. Polychlorinated biphenyls and organochlorine pesticides in Great
Lakes precipitation. J. Great Lakes Res. 5, 61-68.
Strandberg, B., N. G. Dodder, I. Basu and R. A. Hites. 2001. Concentrations and spatial variations of
polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environmental Science
and Technology 35(6): 1078-1083.
Summer K. H., Klein D, and Greim H. (1996). Ecological and Toxicological Aspects of Mono-and
Disubstituted Methyl-, Butyl-, Octyl-, and Dodecyltin Compounds. GSF Forschungazentrum fur Umwelt und
Gesundheit (internal report)
Swackhamer, D.L. and D.E. Armstrong. 1986. Estimation of the atmospheric and nonatmospheric contributions
and losses of polychlorinated biphenyls for Lake Michigan on the basis of sediment records of remote lakes.
Environ. Sci. Technol. 20:879-883.
Swain, W., T. Colborn, C. Bason, R. Howarth, L. Lamey, B. Palmer and D. Swackhamer, 1993. Exposure and
effects of airborne contamination. A report for the U.S.EPA, University of Minnesota, Minneapolis, Minnesota.
109
Thomann, R.V., J.P. Connolly and T.F. Parkerton, 1992. An equilibrium model of organic chemical
accumulation in aquatic food webs with sediment interaction. Environ. Toxicol. Chem. 11: 615.
Tomy, G. T., G. A. Stern, W. L. Lockhart and D. C. G. Muir. 1999. Occurrence of C10-C13 polychlorinated n-
alkanes in Canadian mid-latitudes and Arctic lake sediments. Environmental Science and Technology 33: 2858-
2863.
Turk, J.T., Taylor, H.E., Ingersoll, G.P., Tonnessen, K.A., Clow, D.W., Mast, M.A., Campbell, D.H., Melack,
J.M. 2001. Major-ion chemistry of the Rocky Mountain snowpack, USA, Atmospheric Environment, v. 35, no.
23, p. 3957-3966.
U.S. EPA 1998. Report to Congress - The Incidence and Severity of Sediment Contamination in Surface Waters
of the United States. Washington http://www.epa.gov/waterscience/cs/congress.html.
U.S. EPA, 2002a. The Great Lakes of North America. Great Lakes National Program Office, Chicago.
http://www.epa.gov/glnpo/
U.S. EPA 2002c. Great Lakes Monitoring -Contaminated Sediments Program. Great Lakes National Program
Office, Chicago. http://www.epa.gov/glnpo/sediments.html
U.S. EPA Workshop 2001. Record of the US EPA Great Lakes National Program workshop on emerging
contaminants for fish contaminant monitoring, March 6 2001, Chicago, IL.
http://www.epa.gov/glnpo/monitoring/prog_projects/new_emergcontaminants.html
U.S. NPS 2001. Snowpack and Surface Water Related Citations, United States National Park Service,
Washington. http://www.aqd.nps.gov/ard/aqmon/air_toxics/snow_citations.htm
USGS 2002. U.S. Geological Survey, National Research Program: Effects of Toxic Substances on Aquatic
Communities. Department of the Interior, Washington. http://water.usgs.gov/nrp/proj.bib/leland.html
Wania F., Mackay D. 1996. Tracking the distribution of persistent organic pollutants - control strategies for
these contaminants will require a better understanding of how they move around the globe. Environ. Sci.
Technol. 30, 390A-396A.
WHO, 1984. Environmental Health Criteria 34: Chlordane. World Health Organization, Geneva.
WHO, 1989a. Environmental Health Criteria 83: DDT and its derivatives - environmental aspects. World Health
Organization, Geneva.
WHO, 1989b. Environmental Health Criteria 91: Aldrin and dieldrin. World Health Organization, Geneva.
www.ecosuperior.com/pages/wabigoon
110
4. MAJOR PATHWAYS OF TRANSPORT
This chapter is to provide a basic review of the processes and pathways in North American environment. The
region is essentially a large land mass with huge freshwater resources. The origin, movement and fates of
containments in the region are determined primarily by the nature of pathways through the atmosphere, the
terrestrial compartment and fresh water systems, including sediments. Though other pathways, particularly the
oceans and ground water, have been studied to some degree, they play a relatively minor role.
Taking the atmosphere as the premier pathway, a simple conceptual picture is possible: pollutants entre the
atmosphere in high concentrations, are diffused and diluted over time and distance, are deposited and then are
reconcentrated in aquatic and terrestrial compartments. Effects occur primarily in biota and in humans at the end
of multi-compartment pathways while remediation can occur primarily at the beginning. Generally, air is not an
acutely toxic medium, but rather, a vehicle.
In reality, the pathways followed by individual molecules can be complex. For example, a common pathway is:
emission from a source or volatilization from soil or plant surfaces atmospheric transport deposition to the
terrestrial environment or cultivated land runoff river transport to lakes or estuaries transport to the
ocean. Repetitive revolatilization is also a major factor increasing the complexity of pathways. This process can
occur both from land and water surfaces. In the freshwater compartment, deposition in, and resuspension from
sediments is an important alternative.
4.1 Introduction
The term "pathways of transport" refers to cross-compartment and inter-compartment phenomena operating at a
variety of spatial and temporal scales, linking pollutant releases to environmental compartments and the
subsequent transport and effects of these pollutants on humans and the environment. For UNEP Region II,
emphasis has been placed on transportation by the atmosphere. This is consistent with the approach taken in the
CEC Report on Continental Pollutant Pathways (CEC 1997) but does not suggest that other media are not
important. In the long term, a multimedia approach is critical if we are to understand and protect the whole
ecosystem.
The situation in UNEP Region II is quite different from that which occurs in the North American portion of
Region I, the Arctic. The Arctic is largely made up of islands and, therefore, the marine pathways can be an
important route for pollutants. This is not the case in Region II where the marine environment is entirely on the
east and west coasts with little linkage with the continental land mass.
The long-range transport of air pollutants from UNEP region II, North America, to the North American Arctic
has been well documented in the UNEP Region I Report and elsewhere (Welch et al. 1992). Transport across the
Atlantic Ocean to Europe has also been documented. The parallel evaluation of pollutant transport into UNEP
Region II has not received a great deal of attention compared with the atmosphere transport of PTSs within
region and out of the region.
Within North America two pathways are of particular interest. The first mentioned above is the simple air
pathway from source areas to non-source areas. In some instances, the flow across political boundaries has been
the focus. This pathway is described by many of models including the examples in the next section. In the Great
Lakes and elsewhere, the pathway across the air-water interface has been extensively studied, as noted earlier.
Pollutants cross the air-water interface and entre the water column where they continue on into biota or are
deposited in sediments. In the past, the flow has been into sediments as pollutants were deposited out of
contaminated atmospheres. In recent times, with improvements in air quality, the chemicals stored in lakes are
now moving out of the contaminated sediments and the water column into the cleaner atmosphere, a reversal of
the pathway. In this situation, the sediments have become sources.
The vast majority of contaminants remain in the abiotic environment, in soils, water and sediments. However, a
small fraction can be transferred to biota by direct exposure through water and/or biomagnified in complex food
webs or by maternal transfer. Although the total quantities of OCs in biota are very small compared to the
111
quantities in the abiotic environment, significant bioaccumulation occurs in some parts of the food web resulting
in elevated levels in top predators, including humans.
4.2 Overview of Existing Modelling Programmes and Projects
For most contaminants, the atmosphere is the medium associated with most rapid dispersal. In order to
understand how contaminants move into and out of source areas, it is important to quantitatively calculate the
atmospheric transport in an explicit manner using mathematical models. Having established measurement-
validated atmospheric models, it is possible to estimate the relative importance of different emission sources, to
assess the relative importance of various atmospheric processes, and to evaluate the effectiveness of various
emission reduction measures. The challenges associated with such model development are formidable, requiring
an extensive knowledge of the physical and chemical properties of a given contaminant, along with
comprehensive emission inventories distribution of established accuracy, including the magnitude and spatial
distribution of sources, adequate simulation of physical and chemical processes in the atmosphere, and accurate
ambient measurements for validation. On a regional or continental scale, such information is available for only a
handful of PTSs, constraining the current extent of model development. It is clear that validated models can be
invaluable tools in advancing both our knowledge of the atmospheres and in assessing the effectiveness of
mitigation options but the ancillary activities necessary to support them must be sustained and enhanced.
The study of atmospheric mercury processes illustrates the variety of modeling techniques that have been
developed in the last decade. Currently four types of models are largely in use: (1) Lagrangian modeling, (2)
Eulerian modeling, (3) receptor modeling and (4) mass balance:
1. Lagrangian models are usually formulated under assumptions of simplified turbulent diffusion, no
convergent flows and no wind shear. In these models only first-order chemical reactions can be treated
rigorously. Their advantage lies in the fact that they require less computational resources and can facilitate
an understanding of problems that do not require descriptions of interactive non-linear processes (European
Commission 2001).
2. Eulerian models employ extensive gas and aqueous chemical mechanisms and explicitly track
concentrations of numerous species. Usually these models contain modules designed to calculate explicitly
the chemical interactions that move gas-phase species into and among the various aqueous phases within
clouds while determining the aqueous-phase chemical transformations that occur within clouds and
precipitation droplets (European Commission 2001).
3. Hybrid receptor-deposition modeling techniques combine Lagrangian models with physical or/and empirical
process models in order to assess the relative contribution of atmospheric sources to air masses crossing a
particular monitoring site. They are suitable for regulatory purposes when continuous and relatively dense
(spatially) monitoring network data are available (Cohen 2001).
4. Mass balance models are mathematical descriptions of the environment used to gain a quantitative and
qualitative understanding of the behaviour of mercury species throughout different media (i.e. air, soil,
water). These models subdivide the environment in compartments or boxes, which are assumed to have
homogenous environmental characteristics and concentrations. The model then calculates how mercury
species are distributed within that simplified system.
Building on their early applications in the study of acid rain, air quality models have been developed on the
global, regional and local scale. Local scale models are used to predict concentrations and deposition fluxes
downwind of point sources. Regional and global scale models allow the simulation of long-range transport and
atmospheric fate of mercury. This permits the establishment of source-receptor relationships over some
distance, up to a continental basis. Modeling has also been extended beyond consideration of currently active
sources to the determination of the impact of historical or legacy sources such as previous applications of
currently banned pesticides and transport of emissions from decommissioned facilities and waste sites.
There are dozens of models under development at this time. They vary in temporal and spatial scales,
chemistry, number of contaminants, and number of environmental compartments, for example. A complete
review of modeling as it relates to the physical and chemical fate of contaminants in the various compartments
112
of the North American environment is beyond the scope of this report. The examples given here are primarily
atmospheric models. The majority have been used in North America. They illustrate the kinds of models
available and their applications and provide an indication of the extent of modelling activity. In the case of
atmospheric models, a broader range of examples is found in the papers and abstracts from the World
Meteorological Organization Workshop on Modeling of Atmospheric Transport and Deposition of Persistent
Organic Pollutants and Heavy Metals (WMO 2000) and the reports from the ECE Meteorological Synthesizing
Centre East (EMEP/MSC-E 2002)).
Given that the contamination of humans is the ultimate concern - a contamination frequently resulting largely
from ingestion rather than respiration atmospheric modeling is frequently incapable of simulating such a
multi-compartment transport process. In response, considerable resources have been invested in mass balance
modeling, a process that attempts to account for all the significant pathways taken by a particular contaminant to
environmental compartments occupied by edible biota. For large ecosystems on the scale of the Great Lakes, a
multi-year investment of several million dollars is necessary to secure and analyse the tens of millions of
individual data points required for such a balance. Most recently, the USEPA undertook a mass balance for
mercury, PCBs, atrazine (a current use pesticide) and trans-nonachlor in Lake Michigan. The contribution to the
understanding of the various contaminant pathways of exposure to biota has already been considerable even as
the data continue to be mined for further insights (U.S. EPA LMMB).
4.2.1 Large Scale models
Over the last few decades, a simplified multimedia modelling system constructed on the basis of contaminant
fugacity has been developed by D. MacKay and others centered at Trent University in Peterborough Ontario
Canada. With support from the POPSYCLING-Baltic project funded by the European Union, one of its more
recent iterations examined the movement of lindane among various compartments in the Baltic Sea.
Interchanges among numerous atmospheric, marine, sediment and drainage basin compartments were tracked
over the course of decades. While the spatial and temporal resolution of this model is low, they were adequate
in this application to be indicative of the direction of the movement and the alteration in pattern and fate of
selected contaminants subjected to control actions such as usage bans in the basin.( Wania, F. and K. Breivik
2000).
4.2.1.1 BETR Model of Toxic Transport (Continental Scale)
In an amplification of this work, researchers from Berkeley Lab and Canada's Trent University have developed
the BETR model of toxic transport (BETR 2002, MacLeod et al. 2001). They have incorporated into a single
model toxic release data, wind and water current patterns and regional differences such as soil and vegetation.
The model weighs the absorption traits of each region's compartments (air, soil, etc.) against the solubility of a
specific contaminant
The BETR model divides North America into 24 regions by watershed and soil type. Each region is further
subdivided into seven compartments: upper atmosphere, lower atmosphere, vegetation, soil, fresh water, coastal
water, and freshwater sediment. This map tracks a hypothetical release of toxaphene.
The model is on a continental scale with a database of hydrological and meteorological information which
determines how wind, rivers, and coastal water move contaminants from region to region. By overlaying this
transport data onto 168 mass balance equations (seven for each of the 24 regions), the model maps the pathway
and eventual fate of a contaminant as it's transported across North America.
So far, the model has been tested using real-world data associated with toxaphene. The researchers introduced a
hypothetical 10,000 kilogram-per-year release in the lower atmosphere compartment of the Mississippi Delta
region. The model determined that, although the compound spread throughout much of North America, the
highest concentrations accumulated in the Great Lakes, as illustrated in Figure 11.
113
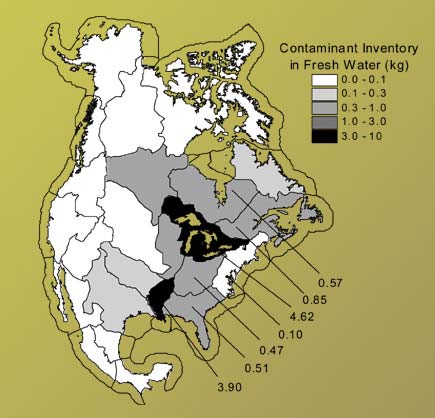
Figure 11: The BETR model predicted consequence of a hypothetical release of toxaphene in the
Mississippi basin.
This model is being further developed at the Lancaster University (Lancaster 2002).
Through the use of such multi-media models, a great deal can be achieved efficiently by fully integrating
processes, observed levels, and trends. In other words, integrating the observed environmental measurements
with the pathways and sources information, so as to provide assessment and feedback on the design and
implementation of future monitoring and process research activities, as well as on management and/or
mitigation measures.
4.2.1.2 A Hemispheric Multi-Component Model for Simulation of HM and POP Long-Range Transport
The Meteorological Synthesizing Centre-East (Moscow, Russia) is an international centre for the co-operative
programme for monitoring and evaluation of long-range transmission of air pollutants in Europe (EMEP). It is a
research institute under the ECE LRTAP Convention (UN-ECE 2002). One of its projects is the development of
a hemispheric multi-compartment model for metals and non-metalic PTSs, in particular, in the atmospheric. The
model uses a horizontal resolution of 2.5º x 2.5º and 10 non-uniform layers in terrain-following coordinates up
to the pressure level of 100 hPa in the vertical direction. As a part of this activity a customized system of
meteorological data preparation has been developed. Several modeling experiments were carried out using the
meteorological information and emission data for 1990. Modeling results were compared with available
measurements and with results of MSC-E regional POP transport model. (EMEP/MSC-E 2002)
To date, the results of POP hemispheric modelling are in a reasonable agreement with those obtained by MSC-E
regional model both in mass balance and in spatial distribution of air concentrations and depositions. The
experiments with PCBs and -HCH showed that some effects known in the literature, such as cold
condensation, are reproduced by the model. The researchers now intend to include the processes of
air/vegetation exchange and the transport by sea currents, and to modify this model for mercury modelling. Most
recently, MSC-East has been co-ordinating the application of selected European and American models to the
estimation of the transport and deposition of mercury.
114
4.2.2 Regional Scale models
4.2.2.1 A Regional Three Dimensional Air-surface Coupled Transport Model.
The BETR model, described in Section 4.2.1.1., is highly complex. Some problems required a narrower focus,
as in the case of lindane in the Great lakes basin. In the basin, lindane levels are relatively high. In Section
3.1.2.3.2, it was shown that the levels are also high in the Prairies. Since 1990, lindane has been used in the
Prairies on canola and in Ontario and Quebec on corn. The source strengths in the Prairies are two orders of
magnitude larger than in Eastern Canada. A regional scale three dimensional coupled atmospheric transport and
soil/air, water/air exchange model has been developed to investigate pesticide transport in North America. This
model has been used to assess the relative importance of lindane source in Canada on the wet and dry deposition
loading to the region in general and to the Great Lakes in particular (Daggupaty and Ma 2002).
The model used made up of two modules, a meteorological component and chemical component. The chemical
component includes the exchange modules for both soils and water surfaces. Using a constructed grided
emissions inventory for the Prairie source area and Ontario and Quebec sources coupled with the meteorology,
the numerical experiment was carried out for the period May 1 to August 31, 1998. The results included:
· There is good agreement between measured and modeled seasonal average air concentrations (pg/m3) at 1.5
m in the Great Lakes basin. The measured values were obtained from the IADN stations (Section 3.1.1.),
· Dry depositions are higher over the land than over the lake surfaces.
· The wet deposition is almost two orders of magnitude larger than the dry deposition.
· Air concentrations are higher over the upper lakes than the lower lakes, due to long-range transport from the
Prairies.
4.2.2.2 A Continental Scale Lagrangian Source Receptor Dioxin Model
The International Air Quality Advisory Board of the International Joint Commission has spearheaded the
application of a Lagrangian transport model to dioxin and mercury in the Great Lakes basin. Under the
leadership of Dr. Mark Cohen of NOAA, the model has successfully estimated the relative impact of the
principal point and area sources of dioxin in the US and Canada to deposition in individual Great Lakes. The
pattern varies with the lake deposition from distant sources is a greater influence on the relatively remote Lake
Superior, while loading to the more heavily industrialized Lake Michigan is associated with nearby sources (IJC
1999). Preliminary results for mercury are currently under examination by the Board, but it is clear that, given
the persistence of some forms of that contaminant in the atmosphere, even more distant sources will prove
influential.
4.2.2.3 A Receptor Model
In many instances the concentrations of a pollutant over an area are know but the location and relative
importance of contributing sources is not known. Receptor modeling techniques are powerful tools that can be
used to locate sources of pollutants to the atmosphere. Identification of pollutant sources is the first step in the
process of devising effective strategies to abate pollutants.
Airborne PCB concentrations are significantly higher in Chicago than in rural areas surrounding the Great
Lakes. This finding suggests that there are significant sources of PCBs in the Chicago region. In an effort to
locate these sources a hybrid receptor model, Potential Source Contribution Function (PSCF), was used (Hsu
and Holsen 2001). Input data were measured PCB air concentrations. The results indicate that areas to the
southwest and to a lesser extent northwest, of downtown Chicago were sources of PCBs. The sources include
wastewater treatment facilities (sludge drying beds), landfills and a large transformer storage yard.
4.2.2.4 A Predictive Model for Gas-Phase Persistent Organic Compounds over Lake Michigan
In the study above by Hsu and Holsen, a model was used to pinpoint the location of PCB sources in the Chicago
area. The next logical step is to determine the relationship between Chicago as a source of PCBs and Lake
Michigan as a potential receptor.
As part of the Lake Michigan Mass Balance study referred to earlier, Hornbuckle1 and Green (2000)
constructed a predictive model for gas-phase persistent organic compounds over Lake Michigan. Using air
temperature, wind direction and atmospheric PCB concentration collected around and over the lake, the model
115
predicts the concentration of PCB congeners (~100) and trans-nonachlor over the lake and gas exchange fluxes.
The model shows that gas-phase PCB and trans-nonachlor concentrations over Lake Michigan vary
considerably as a function of temperature, wind direction and distance from the urban-industrial region of
Southwest Lake Michigan. The Chicago region contributes 2 to 20% of the total deposition of PCB to the lake,
depending on the congener and time of year. For an annual year the total gas-phase deposition of PCBs is
~3200 kg and the Chicago contribution is ~330 kg. The Chicago source behaves like a non-point release by
volatilization from contaminated sites open to the atmosphere and subject to the same temperature fluctuations
as the air temperature. This latter result is consistent with the results from the previous model study (above)
where sludge drying beds were identified as important sources of PCB in the Chicago area.
With regard to trans-nonachlor, the study found that the Chicago source of trans-nonachlor is predominant in the
winter. During the summertime, the source of trans-nonachlor is more diffuse. The trans-nonachlor signal is not
consistent with current use of the pesticide or of seasonal application. This model could not identify the range of
transport.
4.3 The Atmosphere
The atmosphere is the most important pathway relative to ocean and terrestrial/ freshwater pathways for PTSs.
While all of the compartments play a role in transporting these contaminants, the speed of transfer through the
atmosphere suggests that this compartment is particularly important in the regional and global cycling of these
types of compounds.
Generally, the atmosphere contains a relatively small amount of a contaminant compared to the total amount in
other environmental compartments (e.g., for HCH, see Strand and Hov 1996). The importance of the atmosphere
is that it provides a significant mode of rapid transport of contaminants from source areas to receptors. Transport
times of contaminants via air currents are rapid compared to those in the oceans. The time for an air parcel to
completely mix in the troposphere of the northern hemisphere is of the order of six months. In contrast, transport
times of water parcels in northern marine systems are measured in years and decades.
Single and multi-hop pathways
Atmospheric transport pathways can be subdivided into two types: one-hop pathways and multi-hop pathways.
One-hop pathways describe the movement of compounds that are emitted to the atmosphere, transported, and
then deposited to the surface, never to return to the atmosphere. In such cases, the source region of an Arctic
contaminant is simply defined by its source distribution, its lifetime in the atmosphere (governed by removal
processes), and atmospheric circulation. This applies to some organometals and non-volatile, particle-bound
organics, such as benzo(a)pyrene [B(a)P].
With multi-hop pathways, a compound re-enters the atmosphere after initial deposition to the Earth's surface,
and continues over time to move through the environment in multiple hops. Processes by which this can occur
include volatilization from the Earth's surface under temperatures warmer than during initial deposition;
volatilization from water surfaces into a formally dirty but now clearer atmosphere; and re-suspension from
sediments and subsequent volatilization from water surfaces. The later two processes, re-volatilization from
water surfaces and re-suspension from sediments, were discussed earlier concerning the Great lakes (Section
3.1). For multi-hop compounds, the source region is not only defined by atmospheric transport, removal and
circulation, but also by surface processes that control its re-entry into the atmosphere. Mercury, most
organochlorines (OCs), and many PAHs fall into the multi-hop group.
4.4 Freshwater
The most important, large-scale contaminant delivery process to the terrestrial/freshwater compartment is
deposition from the atmosphere. The influence of atmospheric contaminants on freshwater systems starts with
their deposition onto surface waters (lakes, rivers, wetlands) or land surfaces in their catchment areas. While the
total surface area of some lakes, reservoirs, and rivers is large, usually it remains small relative to the total land
surface area (< 1%). The case of the Great Lakes is somewhat different.
Local development has a direct influence on water quality. Given the effects on the environment from
agriculture, refining, manufacturing, construction, and decreased permeability, the quality of water returned to
116
the basin is usually diminished even after treatment. The disposal of industrial and municipal wastewaters is
always a concern. Storm and melt-water runoff, which is usually routed directly to receiving water bodies
without treatment, may be highly contaminated due to broad-cast agricultural chemical, residential garden
chemicals, spills and atmospheric fallout. These factors can exacerbate the problems of identifying and
priorizing effective mitigation measure and providing clean water to basin residents.
4.5 Data Gaps
Emission inventories are a fundamental component of any examination of the occurrence and transport of PTSs
in the environment. They are also an essential input to many models. Their application in models requires a host
of parameters in addition to emission rate, including information on temperature, concentration of contaminant,
velocity, stack or release height, precise geographic location and the presence and concentrations of other
influential contaminants. Although great progress has been made in the last decade to develop regional and
global emission inventories they still remain, overall, inadequate.
Much emphasis has been placed on the pathways for long-range transport of contaminants within the region and
out of the region, particularly into the Arctic. However, the contribution of extra-regional sources to the
regional budget requires further investigation, particularly long-range transport from the west.
The further development and application of regionally specific models for Mexico is urgently needed but must
await the development of a supporting emissions and monitoring infrastructure.
The nature of area sources of PTSs in the central, agricultural sub-region and their pathway to downwind
terrestrial and freshwater compartments needs further attention.
Progress in developing multi-compartment models has been very encouraging. They provide invaluable
guidance on future monitoring and process research activities; management and/or mitigation measures; and the
sensitivity of individual or linked processes. This work must be expanded and applied to a wide range of
ecosystem types in the region.
The importance and consequences of PTSs transport into and out of the mountain ecosystem in Mexico and the
ranges along the west margin of the region need to be better understood.
Collaboration in some parts of the region has been extremely successful, notably in the Great Lakes Basin.
Elsewhere there is a great need for tri-lateral partnerships to address a wide range of common scientific issues
which cannot be resolved by individual laboratories or by sub-regional groups.
4.6 Conclusions
Atmospheric pathways are crucial in the overall context of the movement of contaminants in North America.
However, our understanding of these pathways is very uneven. Movements into and out of the Great Lakes
basin are among the best defined but other pathways, particularly those into the region from the west, influenced
by transpacific movement, those within the mountain ecosystems, and those out of the central agricultural sub-
region are not as well characterized. The pathways in Mexico have in large part not been studied.
With the suite of model available and under development, detailed assessments can be made of both local and
regional movements of PTSs. These assessments are not possible everywhere because of a lack of basic input
data and/or institutional capacity.
A quantified prediction of the ultimate fate of PTSs in the ecosystem would require a multi-compartmentized
approach; models with this capability are current being refining. However, a careful and rigorous examination of
the available data and the desired outcome should precede a commitment to any, even the simplified, models.
The data and resources required to sustain the exercise to a successful conclusion should be in place prior to
embarking on the exercise.
Trilateral collaboration is not sufficiently developed to address regional scale issues concerning the transport of
PTSs.
117
4.7 References
BETR 2002. Berkeley-Trent: A BETR model of toxic transport, Canadian Environmental Modelling Centre,
Trent University, Canada. http://www.lbl.gov/Science-Articles/Archive/EETD-BETR-Krotz.html
Cohen, M. 2001. "The Atmospheric Transport and Deposition of Mercury to the Great Lakes", Progress Report
to the Science Advisory Board and International Air Quality Board, International Joint Commission, Ottawa,
Canada.
Daggupaty, S. M. and J. Ma 2002. A Numerical Investigation of Impact of -hexachlorocyclohexane on the
Great Lakes and St. Lawrence ecosystem, to be presented at the 15th Conference on Boundary Layer and
Turbulence, Am. Met. Soc., Environment Canada, Downsview, Canada.
Eurpoean Commissoin 2001. Modelling the Cycle of Atmphseric Mercury, Chapter 3, Ambient Air Pollution by
Mercury Position Paper, European Communities. http://europa.eu.int/comm/environment/air/pp_mercury3.pdf
EMEP/MSC-E 2002. List of MSC-E publications and reports on HMs and POPs, Meteorological Synthesizing
Centre East, Moscow. http://www.msceast.org/publications.html
Hornbuckle, K.C. and M. L. Green 2000. The Impact of Chicago on Lake Michigan: Results of the Lake
Michigan Mass Balance Study, A white paper prepared for the workshop on "Using Models to Develop Air
Toxics Reduction Strategies: Lake Michigan as a Test Case". University of Iowa, Iowa City. http://www.delta-
institute.org/publications/Hornbuckle-WP-new.pdf
Hsu, Y-K. and T. M. Holsen 2001, The Use of Receptor Models to Locate Atmospheric Pollutant Sources:
PCBs in Chicago, Department of Civil and Environmental Engineering, Clarkson University, New York.
IJC 1999. International Joint Commission,1997-1999 Priorities and Progress under the Great Lakes Water
Quality Agreement. Report to the International Joint Commission, Windsor Ontario, September 1999.
http://www.glfc.org/pressrel/pr092199.htm
Lancaster 2002. European EVn BETR Model, Lancaster University, England.
http://www.es.lancs.ac.uk/kcjgroup/European_model.htm
MacLeod M., D. Woodfine, D. Mackay, T. McKone, D. Bennett, R. Maddalena 2001. BETR North America: A
Regionally Segmented Multimedia Contaminant Fate Model for North America. ESPR - Environ. Sci. Pollut.
Res. 8: 156 - 163.
Strand, A. and Ø. Hov 1996. A model strategy for the simulation of chlorinated hydrocarbon distribution in the
global environment. Water Soil Air Pollut. 86: 283-316.
UN-ECE 2002. Convention on Long-range Transboundary Air Pollution, United Nations Economic
Commission for Europe, Geneva. http://www.unece.org/env/lrtap/
U.S. EPA LMMB. The Lake Michigan Mass Balance Study (LMMB), 2002. U.S. Environmental Protection
Agency. http://www.epa.gov/glnpo/lmmb/project.html
Wania, F. and K. Breivik 2000. Lessons from Modelling Contaminants in Other Large Water Bodies:
Identifying Origin and Time Response of HCHs in the Baltic Sea, White Paper Prepared for the IJC Lake
Michigan Forum Workshop on "Using Models to Develop Air Toxics Reduction Strategies:Lake Michigan as a
Test Case", November 8-9, 2000. Milwaukee, WI. http://www.delta-institute.org/publications/WaniaWP.pdf
Welch et al. 1992. Brown snow: A long-range transport event in the Canadian Arctic. Env. Sci. Tec. 25: 280-
286).
WMO 2002. WMO/EMEP/UNEP Workshop on Modeling of Atmospheric Transport and Deposition of
Persistent Organic Pollutants and Heavy Metals Vol. I (TD No. 1008). GAW PUBLICATIONS, World
Meteorological Organization, Geneva http://www.wmo.ch/web/arep/gaw-pub.html
118
5. PRELIMINARY ASSESSMENT OF THE REGIONAL CAPACITY AND
NEED TO MANAGE PERSISTENT TOXIC SUBSTANCES
5.1 Introduction
The capacity of the North American Region to manage Persistent Toxic Substances (PTSs) is well developed --
more so in Canada and the United States than in Mexico ---- but challenges exist.
For example, with the development of regional (UNECE POPs Protocol) and global (UN POPs Convention)
agreements to manage POPs/PTSs, the attention of regulators and researchers is turning to other chemicals
which exhibit similar properties or criteria for persistence, toxicity and in some cases bioaccumulation. The UN
and UNECE lists are not closed and new substances can be added if they meet the scientific criteria for inclusion
and if ratified by the signing parties. This particular study for the CEC at the behest of UNEP includes additional
substances to the 12 PTSs listed in the UN Convention. While there are reasonably good environmental data on
the 12 PTSs and mercury, there is limited environmental information available on those additional compounds
which are not on the current UN list, making assessment of exposure and risk for the additional compounds
difficult to determine (Environment Canada 2002a).
In Canada, reduced government capacity to assess substances for their toxic properties during the 1990s resulted
in a finding from the Commissioner of the Environment and Sustainable Development (CESD) that "a growing
gap exists between the demands placed on federal departments to provide scientific information on toxic
substances and their ability to meet obligations and respond to emerging issues" (CESD 1999). While, it should
be noted that resources have been restored in recent federal budgets to shore up the Canadian government's
capacity to deal with toxic substances, the same finding holds true in varying degrees for the United States and
Mexico.
As noted in Chapter 2, the sources of PTSs lie primarily within the North American region (regional sources)
with contributions from outside of the region (international sources). Management of the regional
sources/releases of PTSs is the responsibility of the three countries. The management of international
sources/releases of PTSs, which are transported into the North American region primarily via air currents, is a
function of the effectiveness of other countries' programs and international agreements (such as the UN POPs
Convention). North American regional capacity must, therefore, include competence in modeling, monitoring,
tracking, and assessing the atmospheric transport and deposition of PTSs within the region and into the region.
This capacity is important because of the particular concern in several regions in North America (e.g. Great
Lakes, Rocky Mountains) where the elevated concentrations of PTSs found in the environment arise in
significant measure from the long-range atmospheric transport of these substances into these regions. While
significant work has been undertaken across North America, continuing improvement in regional capacity is
needed for modeling, monitoring, tracking, assessing and understanding the phenomenon of the long-range
atmospheric transport of PTSs into the region.
Advances in scientific understanding of how PTSs in the environment affect ecosystem health, including human
health, demonstrate the need to better manage PTSs, in particular, the increasing knowledge of the insidious
inter-generational threats posed by exposure to PTSs (Colborn et al1997). For example, studies on the effects of
PTSs on mothers and their children in the Canada - U.S. transboundary Great Lakes region have lead the
International Joint Commission (IJC) to recommend repeatedly to governments over the years that they improve
their programs for virtually eliminating the releases of PTSs to the environment (IJC biennial reports).
Despite the attention by governments in the region given to the management of PTSs through diverse programs
and the active participation by some NGOs, there is a "sense" that people across the North American region
need a better understanding of why the management of PTSs is vital to their well-being and to the well-being of
others around the world. Regional capacity to communicate this message can be strengthened.
5.2 Monitoring Capacity
Monitoring capacity relates to the ability to measure: (i) the releases of PTSs to the environment from a variety
of sources; (ii) the deposition of PTSs into the environment from processes such as the long-range atmospheric
119
transport of PTSs; (iii) the concentrations of PTSs in the ambient environment e.g. in air, water, biota; and (iv)
the concentrations of PTSs in humans.
5.2.1 Monitoring PTSs releases to the environment
With respect to monitoring and reporting the releases of PTSs and other substances of concern from facilities
and other sources to the environment, the prime mechanisms are North America's pollutant release and transfer
registers (PRTRs). Canada's National Pollutant Release Inventory (NPRI) and the United States' Toxics Release
Inventory (TRI) have been operating for several years, are mandated by law in both countries, and report data
annually on releases and transfers of selected chemicals/substances from industrial and other facilities. These
inventories were extensively discussed in Chapter 2. Mexico is currently setting up a mandatory national PRTR
similar to those in the other two countries; nevertheless, information on some 110 substances in industrial
sectors under federal jurisdiction has been compiled and reported since 1997 under the current voluntary
national PRTR, the Registro de Emisiones y Transferencia de Contaminantes (RETC).
The scope of both the NPRI and TRI has expanded significantly over the years. In the United States, for
example, the number of chemicals/substances included in the TRI has doubled since inception of the program in
1987 to approximately 700. In Canada, the NPRI was launched in 1994 and now reports on almost 300
substances. The objective of both the NPRI and the TRI are to make the data available to the public under
community-right-to-know legislation in both countries, and to empower citizens through this information to hold
companies accountable in terms of how toxic chemicals are managed in their communities. The published data
serve as a rough indicator of progress in reducing the releases/transfers of these substances to the environment.
An increasing number of PTSs are being added on a regular basis to both inventories for monitoring and
reporting. For example, in the United States, a recent innovation by the EPA has been the reporting in 2000 of a
separate category in the TRI devoted to on-site and off-site reported releases for facilities in all industries for
`persistent, bioaccumulative and toxic chemicals'. Many of the chemicals/substances of interest in this UNEP
regional assessment are on the TRI list that includes aldrin, chlordane, dioxins/furans, heptachlor,
hexachlorobenzene, and mercury and its compounds. A separate category on `metals and metal compounds' is
also included in the 2000 TRI (EPA 2000a).
A modification to Mexico's environmental protection law was approved in December 2001, in which industries
will be required to report data on a wide variety of pollutants that are released to the environment. Reporting is
scheduled to begin in the first quarter of 2003, for 2002 information, which will go into a register available to
the public. Included will be releases to air, water and land as well as transfers off-site. Even so, without the force
of law, 117 facilities in Mexico voluntarily provided data in 1999 (CEC 2002a).
The CEC tracks industrial pollution across North America by both chemical/substance and facility and publishes
an annual report entitled Taking Stock, based on information that it receives from the three national PRTRs
mentioned above. The 1999 Taking Stock report presents the first five-year overview of releases and transfers in
the region and provides an opportunity to spot trends and measure progress. PTSs are a part of this reporting.
Measures are being taken to improve the comparability of data and information between the three PRTRs
including the manner in which data on PTSs (which are bioaccumulative such as mercury, dioxins and lead) are
collected, subject to the technical, economic, and regulatory capacities of each country (CEC 2002b).
Other monitoring programs in existence in each country, in transboundary regions, and across the entire region
have been helpful in augmenting information on the releases of PTSs to the environment. In each country, useful
information on releases is obtained from industries reporting on their accomplishments to reduce PTSs through
mechanisms such as Responsible Care by the chemical industry, or through voluntary agreements between
industry and the federal and provincial/state governments. In border regions, for example, the Canada-U.S.
Binational Toxics Strategy has improved monitoring capacity and information on selected PTSs releases to the
environment. Across the entire region, the CEC's tri-national Sound Management of Chemicals (SMOC)
program has stimulated the production of pollutant release and transfer information for several PTSs in order to
develop North American Regional Action Plans (NARAPs). For example, the current NARAP under
development for dioxins and dioxin-like compounds has resulted in inventory updates of these compounds in all
three countries.
120
5.2.2 Monitoring the deposition of PTSs to the environment from short/long-range atmospheric transport
Loadings of toxic substances from atmospheric deposition are estimated in a number of key ecosystems across
North America. Capacity to undertake this type of monitoring, modeling and tracking of PTSs across the region
is improving rapidly, particularly in Canada and the United States, with advances in scientific expertise,
methodologies, modeling, peer review, and quality assurance. In some cases, these activities involve the
inclusion of selected PTSs in existing networks designed to monitor other pollutants such as volatile organic
compounds (VOCs) and acidifying substances.
In the Great Lakes ecosystem, for example, Canada and the United States have cooperated in establishing an
Integrated Atmospheric Deposition Network (IADN) that has been in operation since 1990. IADN is a system of
monitoring stations created under the Canada-U.S. Great Lakes Water Quality Agreement with specific goals to
identify airborne toxic substances and their sources, to track their movements, and to define trends in their
atmospheric deposition to the lakes. This network was discussed extensively in Chapter 3. IADN has been
referred to by a panel of independent scientific experts in 1997 as "a leading international effort in the
assessment of the role of the atmospheric impacts of persistent, toxic substances on aquatic systems"
(Environment Canada - U.S. EPA 1996).
EPA is collaborating with a number of institutions (e.g. the National Oceanic and Atmospheric Administration
or NOAA) to identify and evaluate the long-range transport of certain PTSs into the United States. For example,
the Agency is collecting measurements at Cheeka Peak, Washington, to identify and evaluate the trans-Pacific
transport of mercury and other pollutants in air masses that originate in Asia (e.g. forest fires in Indonesia). EPA
will also begin collecting similar measurements at a high altitude site on Mauna Loa, Hawaii. Among other
things, the study will enhance EPA's understanding of the transformation of elemental mercury to reactive
gaseous mercury. Other EPA initiatives include expanding the National Dioxin Air Monitoring Network
(NDAMN) to investigate the potential for long-range transport of dioxins, furans, and dioxin-like PCBs from
Asia to North America (EPA 2000b).
Similar monitoring capacity is needed in Mexico.
Finally, monitoring and modeling capacity is necessary to be able to do mass balance studies. Mass balance
studies are increasingly being done in the region because they are useful tools for understanding and assessing
the movement of PTSs in ecosystems ---- hence improving the region's capacity to manage PTSs. Mass balance
is based on the principle of `conservation of mass', that is, the amount of a pollutant entering a system should
equal the amount of pollutant leaving, trapped in (sinks), or chemically changed in the system. The mass balance
approach integrates environmental monitoring, load estimation of the PTSs, and research efforts within a
modeling framework that is compatible with both scientific and management objectives.
The Lake Michigan mass balance is one example of such a study with the objective of understanding the
complex pathways of PTSs (PCBs, atrazine, trans-nonachlor, and mercury were studied) through the system so
that effective management strategies can be designed to reduce the threat from toxic chemicals. The model
design for the Lake Michigan Mass Balance was based on those used for the Green Bay Mass Balance study and
consistent with those used in other studies such as the Chesapeake Bay Watershed Model (US EPA 2002c).
5.2.3 Monitoring PTSs concentrations in the ambient environment
Current data substantiate that, despite prohibitions, restrictions and regulatory controls on releases, many PTSs
are still ubiquitous in the environment, and at levels that may be of concern for both human and wildlife. Human
exposure occurs mainly through the food chain and for the most exposed populations, exposure is probably due
to consumption of contaminated fish. Potential risk and health consequences are of particular concern for human
populations which have increased exposure such as subsistence fishers or which have increased susceptibility
such as developing embryo/fetus, nursing infants, and children (EPA 2000d).
As reported in Chapter 3, levels and concentrations of PTSs in the ambient environment have been monitored
fairly extensively in North America. The International Joint Commission and its Advisory Boards, have reported
at length on concentrations of contaminants such as PCBs and mercury found in fish and other wildlife in the
Great Lakes ecosystem (IJC Biennial reports); wildlife experts have postulated that elevated concentrations of
mercury in loons in Atlantic Canada and the North-eastern states might be a significant reason for the apparent
121
decline in their numbers in recent years (Evers et al. 1998); and research in water and biota in Canadian Rocky
Mountain ecosystems has illustrated the "grasshopper" effect, showing elevated concentrations of certain PTSs
in these ecosystems (Blais et al. 1998).
There is other work in progress. The U.S. EPA is conducting a 4-year National Fish Tissue Study which is
expected to produce a wealth of data, including defining the prevalence and background levels of persistent
bioaccumulative toxic (PBT) chemicals in fish (US EPA 2000b); and the United States and Canada are
embarked on a significant initiative to identify environmental indicators which can measure the success of the
two countries in achieving the goals of the Great Lakes Water Quality Agreement, including the reduction of
PTSs concentrations in the basin.
Of interest is the evolution of Canada's Ecological Monitoring and Assessment Network (EMAN), a broad
based scientific partnership among stakeholders from all sectors. EMAN's primary goal is to bring together
independent monitoring activities to better understand the priority stressors that are affecting ecosystems.
Linkages are being forged with international monitoring and research networks in studying hemispheric air
pollution, including the long- range transport of PTSs (Environment Canada 1998). For example, on the mercury
issue, EMAN has worked with the CEC, and has been instrumental in promoting international partnerships such
as the Canada-US mercury deposition network, and the Americas Mercury Deposition Mercury Network with
sites proposed for Canada, the U.S. and Mexico.
Though these monitoring efforts are both comprehensive and wide-ranging, and some explicitly includes
Mexico, without adequate staff competence, laboratory facilities and field research capacity, Mexico cannot
participate effectively.
Environmental Effects Monitoring (EEM) is another scientific tool that assesses the effects of effluent from
industrial or other sources on fish, fish habitat and the human use of fisheries resources. EEM can be used,
therefore, to assess the adequacy of regulations in protecting aquatic environments, as has been required of the
Canadian pulp and paper industry to determine whether dioxins/furans have been virtually eliminated from pulp
and paper mill effluents; similar requirements are being drawn up for the mining industry under the Metal
Mining Liquid Effluent Regulations (Environment Canada 2002b).
Clearly, there is considerable monitoring being done in the North American region on many aspects of the issues
posed by PTSs, particularly in Canada and the United States. From a North American regional perspective,
however, the work is patchy and disconnected in that it reflects and focuses mainly on problems that have arisen
in the three countries respectively from certain PTSs. While that is important, opportunities for cooperative
work/synergy are not being fully realized. The desirability of the cooperative approach seems self evident given
the nature of PTSs in terms of their ability to be transported through the atmosphere for long distances. A good
start has been made with the very effective CEC program on the Sound Management of Chemicals (SMOC).
Much more needs to done as described in the CEC's NARAP on Environmental Monitoring and Assessment
which was adopted by the CEC Council in June 2002 (CEC 2002b).
The CEC's NARAPs on various specific substances such as PCBs, chlordane, mercury (all being currently
implemented), dioxins/furans, hexachlorobenzene, lindane (under development) and lead (under assessment),
demonstrate a long-term commitment to cooperative regional action on PTSs. As stated in the NARAP on
Environmental Monitoring and Assessment, "... the sharing and transfer of information and best practices are
seen as important means of enhancing national capacity for the sound management of these substances. Other
important elements and outcomes of these cooperative initiatives include collaboration and cooperation in the
measurement, monitoring, modeling, research and assessment of selected PTSs and toxic substances in
environmental media. For instance, the NARAP on mercury includes specific obligations related to the
monitoring, research, modeling and assessment of mercury in the environment and its implications for human
health and the environment. Such cooperation is considered essential to improve the quality, comparability,
availability and relevance of the `environmental information' needed to make informed and responsible
decisions throughout NARAP implementation." The NARAP on Monitoring and Assessment offers, therefore,
an opportunity to help build capacity in the region, particularly as it is to encompass a strong focus on children's
122
health. This includes any process leading to the enhancement or strengthening of a knowledge or skill base
through the transfer, reciprocation or exchange of information and personnel between organizations.
Improving monitoring and assessment capacity in the region, particularly in Mexico, will require substantial
infusions of funding. To be successful, it will also require strong support from the monitoring and science
communities and the public in all three countries.
It should be noted, however, that government funding throughout the region for fundamentally basic programs
in aid of good science and research has suffered in recent times because they are not seen as "politically" high
profile. Perhaps, the increasing emphasis on human health, particularly children, and the threats posed to future
generations by PTSs may serve to give the necessary impetus for appropriate funding and resources. Efforts
should be made also to explore funding from international funding agencies, such as the GEF, for building this
particular capacity in Mexico to meet that country's special needs.
The current situation in Mexico may be illustrated by the following example. The only Mexican Official
Standard applicable to water is NOM-127-SSA1-1994, relating to environmental health, water for human use
and consumption, and allowable limits of quality and treatment for potable water supply. Table 8 presents the
NOM requirements for the analysis of the drinking water supply for a number of PTSs.
Table 5: NOM requirements for the analysis of PTSs in drinking water supplies
Pesticides
Micrograms/L
Aldrin and dieldrin (separate or combined)
0.03
Chlordane (total isomers)
0.30
DDT (total isomers)
1.00
Gamma-HCH (lindane)
2.00
Hexachlorobenzene 0.01
Heptachlor and heptachlor epoxide
0.03
Methoxychlor 20.00
2, 4-D
50.00
The CNA, responsible for providing authorizations for drinking water extraction, does not have the necessary
laboratory infrastructure enabling it to monitor these substances. The most serious case is the deficiency in
monitoring bodies of water that receive industrial wastewater and/or agricultural runoff, which have a high
probability of containing such substances.
Finally, in the effort to improve capacity in the region on monitoring and assessment, it should be noted that all
three North American countries are members of the OECD. The OECD Council decision on implementing Good
Laboratory Practice should be revisited by experts in the three countries to determine how that decision can be
useful in improving laboratory methodology relating to quality assurance/control (QA/QC) in analyzing and
measuring contaminants in a reliable, consistent and comparable manner across the region.
5.3 Existing regulations and management structures
Federal legislation, regulations and management structures apply to toxic substances in all three countries. Table
6 summarizes the management regime for the PTSs in this regional assessment.
Table 6: The Management Regime for PTSs in North America
Name of PTS
Canada
United States
Mexico
Aldrin
voluntarily withdrawn 1990 banned 1987
prohibited
Chlordane registrationdiscontinued restricted use 1988
registration withdrawn*
1990
DDT
voluntarily withdrawn 1985
banned 1972; products with
restricted use **
more than 0.1% banned
123
Name of PTS
Canada
United States
Mexico
1986 (Dicofol)
Dieldrin
restricted use 1987
banned 1971
prohibited
Endrin
major use restrictions mid
banned (date?)
prohibited
1970s
registration ended1990
Heptachlor
major use restrictions 1976
banned (date?)
prohibited
registration ended 1985
HCB
Pesticide: registration not
registration cancelled 1984
prohibited
renewed 1976
Chemical: prohibited 2003
except when incidentally
present in a product, in a
concentration not exceeding
20 ppb
Toxic Substances
Management Policy (TSMP)
Track 1 Substance, targeted
for Virtual Elimination (VE)
Mirex
never registered
use cancelled 1988
prohibited
Toxaphene
withdrawn 1982
banned 1982; use ceased
prohibited
1986
PCBs
severely restricted 1977;
new uses cancelled 1970;
new uses prohibited; closed
open and new uses banned,
closed use allowed; a wide
use allowed; a wide range of
closed use allowed; a wide
variety of federal
regulations & requirements
variety of regulations and
regulations and
for PCB use, information,
guidelines, federal and
requirements for PCB use,
storage, treatment &
provincial, for PCB use,
information, storage,
disposal, incineration/air
information, storage,
treatment & disposal,
emissions,
treatment & disposal,
incineration/air emissions,
shipments/export, and PCB
incineration/air emissions,
shipments/export, and PCB
waste.
shipments/export, and
waste.
wastes containing PCBs.
Dioxins
regulated for pulp and paper Regulated for pulp and
mill effluents under CEPA
paper mills
and are subject to the
Canada-Wide Standard
Process
Furans
regulated as per dioxins
As above for dioxins
HCH
PCP
registered under the Pest
A restricted-use pesticide
Products Control Act
under the Federal
Strategic Options Report
Insecticide, Fungicide, and
finalized under CEPA
Rodenticide Act ("FIFRA")
Regulated under the
Resource Conservation and
Recovery Act, Clean Water
Act and the Safe Drinking
Water Act
PAHs
Controlled through the
Strategic Options Process
124
Name of PTS
Canada
United States
Mexico
Org. Mercury
Chlor-alkali mercury release Chlor-alkali plant releases
Compounds
regulations under CEPA &
regulated
the Fisheries Act and is
subject to the Canada-Wide
Standard Process
Org. Tin
restricted use
restricted use 1988
Compounds
(TBT)
Org. Lead
Tetra-ethyl lead banned in
Tetra-ethyl lead banned in
Compounds
on-road motor gasoline
on-road motor gasoline
* In 1996, Mexico informed the importing and formulating company of the chlordane-based termicide of its
intent to suspend imports and to cancel the product's registration once the remaining stocks in the country
were used up, an action under the CEC NARAP on Chlordane (non-legal document).
** Reductions in use to be phased in as per the North American Regional Action Plan on DDT. The NARAP
does not have force of law.
Many of these actions were through voluntary removal from the market. The end of registration date
indicates the timing of the regulatory action taken.
5.3.1 Canada
In Canada, nine pieces of federal legislation apply to toxic substances. New and existing substances are
primarily regulated under the Canadian Environmental Protection Act (CEPA), the Pest Control Products Act
(PCPA), the Food and Drugs Act (FDA), the Hazardous Products Act, the Fertilizers Act, the Fisheries Act and
the Feeds Act. CEPA deals with toxic substances not regulated under any other piece of federal legislation. Non-
regulatory approaches are also used, where appropriate, to manage releases of toxic substances to the
environment.
The approach the federal government uses under CEPA sets out two tracks for the management of toxic
substances, namely (i) virtual elimination from the environment of toxic substances that result from human
activity and that are persistent and bio-accumulative (that is, PTSs), and (ii) management of other toxic
substances of concern, during their life cycles, to prevent or minimize their release to the environment. The tools
to manage toxic substances under CEPA include pollution prevention plans, environmental emergency plans,
virtual elimination plans, regulations, economic instruments, guidelines, objectives, and codes of practice. A
joint management committee of senior level officials from Environment Canada and Health Canada oversee the
implementation of CEPA programs pertaining to toxic substances.
CEPA's legislative framework for assessing and managing toxic substances underwent major amendments in
1999 and upcoming amendments to the PCPA will strengthen health and environmental protection and increase
the transparency of the registration system. Health Canada is also upgrading its infrastructure to support stronger
management of toxic substances, including making better use of cutting-edge science and new information
technologies.
Environment Canada has also used non-regulatory or voluntary approaches, such as the Accelerated Reduction
and Elimination of Toxic Substances (ARET) initiative, to reduce the releases of toxic substances to the
environment.
Provinces play a partner role in the management of toxic substances, generally through the federal-provincial
forum of the Canadian Council of Ministers of the Environment (CCME) where Canada-wide standards are
formulated and then implemented by the level of government most suited to the task. The environment is a
provincial responsibility. Transboundary effects and contamination are a federal responsibility. For example, in
Ontario, toxic substances are regulated under a number of instruments in addition to the Canada-wide standards.
The PCPA regulates the registration of all products imported, manufactured, sold, or used in Canada to control
pests. It states that no pest control product will be registered until all associated health or environmental health
risks have been deemed acceptable and the product has been shown to serve a useful purpose.
125
The FDA applies to all food, drugs, cosmetics and medical devices sold in Canada, and new drugs cannot be
marketed without the approval of Health Canada confirming that their manufacture and sale comply with FDA
regulations. In recent years, research is showing low concentrations of some of these "new" chemicals in the
environment and studies are now being ramped up to understand the impact of these substances on the
environment.
While cancer has historically been the focus of assessments, recent research suggests that significant, non-cancer
health impacts can arise from long-term, low-level exposure to a mix of substances. Governments in Canada are
therefore trying to determine whether the current scope of their research and regulatory activities is appropriate.
5.3.2 United States
In contrast to Canada, the United States has a more centralized system for managing toxic substances. The U.S.
Environmental Protection Agency (EPA), for example, is mandated to act as `watchdog' for both the
environment and human health. The U.S. system embraces independent institutions such as the National
Academy of Sciences, the Atlanta Toxic Substances Disease Registry, a `superfund' to spur the clean up
environmental disasters, and programs to evaluate the impact of environmental factors on children's health. The
Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) are
key pieces of legislation for managing toxic substances.
In addition to managing PTSs in use, EPA exercises its authority under TSCA to screen new chemicals intended
for industrial markets in an effort to prevent the introduction of new PTSs that are bioaccumulative (PBTs). EPA
has also taken steps to establish a similar policy for pesticides under FIFRA. Using pre-manufacture
notifications or PMNs submitted by industry for new chemicals, EPA evaluates the information to ascertain
whether they possess PBT characteristics. Based on these reviews, EPA can stop the production of these
chemicals until their prospective manufacturers can prove that they will not pose an unreasonable risk if released
into the environment. An innovative tool being tested by EPA is the PBT Profiler that estimates whether a
particular chemical possesses PBT characteristics based on chemical structure and physical and chemical
properties.
EPA's PBT Program currently focuses on a list of 12 priority PBT pollutants, namely: aldrin/dieldrin, alkyl-
lead, benzo(a)pyrene, camphechlor (toxaphene), chlordane, DDT (+DDD & DDE), dioxins/furans,
hexachlorobenzene, mercury and mercury compounds, mirex, octachlorostyrene, and PCBs. For each
substance/chemical, a national action plan has been, or is being, developed with priority actions spelt out. These
action plans are consistent with international efforts involving Canada, Mexico and the United Nations.
EPA applies a holistic and integrative approach to managing PBTs including every Agency tool available --
regulatory, compliance assistance, enforcement, research, voluntary actions and international negotiations.
Environmental agencies in the states also play an important role in managing toxic substances. Interested
stakeholders and the public are given opportunity to comment and input at critical stages of development of PBT
policy, rules, and programs, and notable accomplishments have been achieved in partnership with some
associations. The American Hospital Association and the American Nurses Association, for example, in
collaboration with EPA have created a voluntary program called Hospitals for a Healthy Environment or H2E
that has achieved notable reductions in mercury releases from hospitals. This is but one example that illustrates
the willingness of public interest groups to cooperate with regulatory agencies in all three countries to help
reduce the releases of PTSs to the environment.
5.3.3 Mexico
The legal framework governing chemical substances continues to be one of the most complicated and confusing
areas of law, due to the existence of countless varying provisions applicable to PTSs in Mexico.
The analysis of these laws shows duplication of effort among them, as seen in Table 5-3, which makes
application and enforcement difficult. There is also a lack of systematization in the integration of provisions,
complicating the objectivity thereof due to shortcomings and overlapping in the allocation of enforcement
responsibilities.
126
Table 7: The Legal Provisions Relating To Handling of PTSs in Mexico
Competencies
/
or
d
ation
n
an
t
a
l
d
Provisions
Field of Application
liz
n
t
i
on
i
on
g
t
a
h
t
a
t
i
o
n
a
t
i
on
t
i
on
la
onmen
i
stration
or
sportatio
act Assessment
r
a
t
i
n
o
riza
t
r
o
l
p
n
xport
mmercia
e
gu
nvir
pe
uth
versig
on
Reg
Product
I
m
E
Co
Tra
R
E
I
mp
O
A
O
C
General Law of Ecological Balance and Hazardous waste, substances and
materials
X X X X X X
Environmental Protection (LGEEPA)
Pesticides, fertilizers and toxic substances
X
X
X
X
Hazardous waste, substances and
materials
X X
General Health Law
Pesticides,
fertilizers
and
toxic
substances
X X X X X X
Hazardous waste, substances and
materials
Federal Law on Plant Health
Pesticides,
fertilizers
and
toxic
substances
X X X X X
Hazardous waste, substances and
materials
Federal Copyright Law
Pesticides, fertilizers and toxic substances
X
X
Hazardous waste, substances and
materials
X X
Law of National Waters
Pesticides, fertilizers and toxic substances
X
X
Hazardous waste, substances and
materials
X X X
Federal Penal Code
Pesticides, fertilizers and toxic substances
X
X
X
Hazardous waste, substances and
materials
X
Navigation Law
Pesticides,
fertilizers
and
toxic
substances
X
Federal Regulations for Workplace
Hazardous waste, substances and
materials
X X
Safety, Hygiene and Environment
Pesticides, fertilizers and toxic substances
X
X
Regulations for the Land Transport of
Hazardous waste, substances and
materials
X X
Hazardous Materials and Waste.
Pesticides, fertilizers and toxic substances
X
X
127
5.3.4 North American Regional and International Cooperation
There are provisions in CEPA that require Canada to cooperate with the United States and other OECD
countries, including Mexico, to exchange information on prohibited and severely restricted toxic substances, and
to re-examine any substance that an OECD country decides to prohibit for environmental and health reasons.
Environment Canada and the EPA have enjoyed a productive relationship over the decades and are progressing
towards an even closer working relationship in this direction. Mexico is playing an increasing role in this
regional partnership, particularly through the efforts of the CEC, in managing and preventing PTSs from
entering the North American environment.
There are tens of thousands of chemicals in use, however, in the three countries, including many toxic and
potentially toxic substances. Managing these substances is a huge and complex undertaking. Assessments of
new chemicals and reassessments of products currently in use pose challenges to the management capacity of
the region's governments. One approach to the assessment backlog is to increase efforts to harmonize with each
other and other OECD countries. The OECD, through its Chemicals Programme, is undertaking several joint
efforts to foster the mutual acceptance of data related to the assessment of chemicals. For example, all OECD
countries have agreed to accept safety data developed in other member countries to support the risk assessment
process. As well, there are a series of OECD initiatives dealing with registration, notification, cooperative
assessments, and assessment and classification of best practices addressing new and existing chemicals and
pesticides. These efforts will determine the extent to which countries will accept other nations' assessment
information and potentially, their regulatory decisions. While these cooperative initiatives will not remove a
country's authority to approve or reject a substance, it is hoped that increased efficiencies will reduce costs for
businesses and governments.
It would be remiss if recognition were not given to industry's voluntary efforts to assure that appropriate hazard
information is available for high production volume chemicals, under the U.S. High Production Volume
Challenge program and to the OECD Screening and Information Data Set (SIDS) program. The assessment
techniques being refined in the HPV and SIDS programs (e.g., read-across, QSARs) are demonstrated methods
of reducing burden on government and industry, speeding assessments, and reducing the need for new animal
testing.
Finally, increasing efforts are being made to improve efficiency by coordinating regional activities with existing
workgroups and other organizations such as the Technical Working Group on Pesticides (United States-Canada
Free Trade Agreement), the Ad Hoc Working Group on Persistent Organic Pollutants (Inter Organizational
Program for the Sound Management of Chemicals, IOMC), the Intergovernmental Forum on Chemical Safety,
and the United Nations Economic Commission for Europe/Long Range Transport of Air Pollutants
(UNECE/LRTAP) Ad Hoc Workgroups on POPs and Heavy Metals.
5.4 Status of Enforcement and Compliance
All three countries have enforcement capacity to administer the laws against pollution of the environment from
PTSs. Federal agencies and provincial and state agencies in the three countries cooperate in enforcing these
laws, often through agreements which delegate the responsibility to the level of government most suited to
administer these laws as described, for example, in the harmonization agreements between the Canadian federal
government and the provincial governments. Some jurisdictions have placed a greater emphasis on industry self-
regulation; however, audits of performance of those responsible for addressing non-compliance of
environmental laws have indicated less than satisfactory enforcement in many instances.
The law enforcement record of governments in the North American region has come increasingly under
scrutiny, particularly by the ENGO community, in the last decade or so, since the advent of NAFTA and the
North American Agreement on Environmental Cooperation (NAAEC). One of the principal aims of the NAAEC
is the promotion of effective enforcement by the Parties of their domestic environmental legislation.
Accordingly, the NAAEC provides, under Articles14 and 15, a means by which anyone living in any of the
three countries of North America may bring the facts to light concerning the enforcement of environmental
legislation on the books of any of the three countries. Increasing numbers of citizens submissions are being
lodged with the CEC alleging improper, or lack of, enforcement of environmental laws in all three countries,
128
albeit with mixed success to date. In addition, there is an increasing public profile being given by ENGOs to
what they perceive as inadequate law enforcement by governments. Legal action in the courts has increased in
large measure because of information now accessible as a consequence of community-right-to-know legislation
in the United States and Canada.
As with other environmental programs, capacity for enforcement action varies among the three countries. The
CEC's on-going project on Enforcement and Compliance Capacity Building supports initiatives to enhance the
Parties' respective capacities for effectively enforcing their environmental laws and regulations. Pollution
control, tracking and enforcement is being addressed on matters identified by the Parties as priorities; in 2001,
an initiative has been undertaken to enhance regional capacity for enforcing North American laws and
regulations implementing the Montreal Protocol on Substances that deplete the Ozone Layer. Since the
management of PTSs are increasingly a matter of regional concern and cooperation, as demonstrated by the
ongoing process of developing and implementing NARAPs, these substances may become an increasing priority
for the Enforcement and Compliance Capacity Building agenda by considering such aspects as innovations in
regulations, economic instruments, voluntary or non-regulatory initiatives, and enhancing public participation in
processes for establishing and enforcing environmental standards.
5.5 Alternatives or Measures for Reduction
Substitution and alternative technologies to reduce and/or eliminate the use and release of industrial and
commercial products that are persistent and toxic have been developed and widely used in the North American
region for at least two decades.
Many of the complex, long-chain, chlorinated pesticides used in agriculture have been replaced by other
products that are not as harmful to the environment. PCBs are no longer being manufactured and are being
replaced by more benign products. Mercury usage is being replaced in many applications, as is lead, particularly
where the risk is high of release into the environment with subsequent exposure to humans through
environmental media and/or the food chain.
In the case of by-products which are released from industrial processes and which are persistent and toxic such
as dioxins/furans, significant success has been achieved through the application of best available technology,
alternative chemicals and processes. In pulp and paper production, emissions of 2,3,7,8-TCCD/TCDF in the
United States and Canada have been virtually eliminated because of the shift from molecular chlorine to
chlorine dioxide. Significant reductions in the release of dioxins from incinerators that burn municipal and
hospital medical wastes have been achieved through improved combustion technology and source separation.
However, source separation appears to be considerably less effective when compared with the technological
solution.
Industry in the North American region has shown itself to be technologically capable of developing alternative
processes or products to replace the uses of PTSs where it has been shown that risk management is not an
option. Removal at source and other pollution prevention techniques have been the preferred course of action,
where feasible. Where PTSs are present in waste streams as a consequence of industrial processes, the region
has also shown itself capable of treating and disposing of these toxic releases in an environmentally sound
manner.
An important driver for new technology is the clear, stated intent of governments to legislate/regulate the
product or release out of existence, either initially or as a backstop to voluntary initiatives by industry.
Technology-forcing regulations have been used extensively in North America to eliminate/reduce the release of
toxic substances to the environment. While there are those who do not favour this approach, it has been
generally successful in achieving environmental objectives.
Pollution prevention and new technology to protect the environment is big business in North America. The
environmental industry keeps growing at a significant pace. Alternatives to the use of PTSs and measures for the
reduction of PTSs to the environment will likely continue to be a focus of attention in the region. This can be
expedited by means of firmer government policy and action on PTSs. The CEC's initiative in facilitating
129
communication among pollution prevention and technology networks in the region is helpful regarding the
exchange of information on alternatives and measures to reduce/eliminate PTSs.
5.6 Technology Transfer
The three countries all participate in the OECD's clean production/technology initiative which includes
encouraging technology transfer. Trade shows in major centers in the three countries regularly exhibit the latest
developments in new technology to reduce and eliminate environmental pollution. Through multi-stakeholder
fora hosted by the CEC, information exchange is facilitated on a variety of issues including the sound
management of chemicals. Pollution prevention programs operate in all three countries, and the CEC's pollution
prevention initiative, although limited in scope, has been a success to date.
The three countries, under the existing NARAPs, have agreed to cooperate in a variety of ways to achieve the
objectives of the NARAPs, including information exchange, joint scientific and technical workshops, and
technology transfer. This practice will continue for new NARAPs as they are developed and implemented.
There are other avenues for technology transfer such as the March 1990 Agreement on Environmental
Cooperation between the Government of Canada and the United Mexican States which can be used. This
agreement allows for, among other things, cooperation on technologies that promote environmental quality and
mitigate environmental damage, environmental training and education, environmental monitoring, and methods
for assessing environmental quality.
In short, there are a number of means by which technology transfer can be achieved, and in this regard, the three
countries could place a specific emphasis on technology transfer initiatives for the reduction and elimination of
PTSs in the region. With regard to Mexico, and particularly with respect to the twelve PTSs identified in the UN
Convention, seeking financial resources from international donor agencies to enable technology transfer appears
to be an appropriate route to take in pursuing the objectives of the Convention.
5.7 Identification of Needs
Regional capacity to improve the management of PTSs in North America requires the following:
· Community education -- a significant community education process is necessary to enhance the impetus for
restricting/eliminating the use of PTSs -- including and involving vigorous and capable NGOs at the
national, state/provincial and local levels. The public health community is one such interest group that has,
and should be encouraged to play a (more) significant role and take greater responsibility in the area of
education as advocates for the public interest (WFPHA 2000);
· Research and monitoring programs national programs need to be enhanced to ensure that information on
current levels and trends of old and emerging PTSs are available in all regions in major environmental
media e.g. water, air, fish, sediments;
· Ecosystem health assessments -- the assessment and understanding of the science and economics of what
PTSs are doing to the health of ecosystems (environmental media, biota and humans) needs to be
strengthened through additional funding for long-term field studies, and support for training. In this regard,
the CEC's Children's Health initiative should be supported over the longer term to collate, make available,
and inform the North American community of the latest developments regarding the effects of PTSs;
· Integrated atmospheric deposition and monitoring networks a wider coverage of integrated monitoring
networks should be established in "vulnerable" ecosystems across the region, particularly in Mexico, to
better define the presence, levels, fate and pathways of PTSs. Implementation of the proposed NARAP on
Monitoring and Assessment will be a useful step in this regard;
· Technology Transfer the management of PTSs needs to be given a higher profile under technology
transfer/exchange agreements between governments in the region, particularly in view of Mexico's special
needs;
· Pollution Prevention greater emphasis should be placed on installing pollution prevention technology (as
opposed to control technology) and practices to restrict/eliminate releases of PTSs. Fiscal incentives by
130
governments might be helpful towards this end as would the provision of incentives in the CEC's pollution
prevention initiative;
· Socio-economic analysis In order to build the fact base and improve decision making, more rigorous
analysis needs to be conducted of socio-economic factors, such as food production and impact on human
health, that may arise as a consequence of taking action to manage PTSs;
· Cooperation efforts should be improved on ensuring compliance with national programs and regulations,
including voluntary measures, in the three North American countries --- harmonized to the extent possible,
while respecting the differing circumstances across the region as reflected in the CEC's North American
Regional Action Plans;
· Substitution enhanced research and development is needed to produce environmentally benign substitutes
to replace PTSs, including the use of alternative technology driven by regulatory rules and other incentives
that stimulate voluntary action.
5.8 Conclusions
Institutional capacity is relatively mature in Canada and the United States but needs to be strengthened in
Mexico. Across the region, additional resources are required from governments, funding agencies and other
partners to establish and sustain the programs required to make meaningful progress in the management of
PTSs.
Public education to improve awareness, training to develop technical expertise, access to financial
capital/resources, and technology transfer and capability to implement these programs can all be enhanced.
While transparency in decision-making on toxic substances has improved, stakeholders in the region say that
there is room for improvement in terms of faster and clearer communication regarding access to information and
regarding input to the management of toxic substances.
Ensuring greater government openness and public involvement in, and across, the three countries would increase
public confidence in the regulation and overall management of toxic substances.
In all the above aspects, and particularly with respect to environmental monitoring of PTSs, Mexico requires
special attention and consideration to help that country meet its commitments.
The CEC should continue to play a facilitative role in helping to achieve common objectives and in establishing
collaborative trilateral approaches in the many programs and initiatives to manage PTSs.
5.9 References
Blais, D. Schindler, D. Muir et al. 1998. Accumulation of persistent organochlorine compounds in mountains in
Western Canada. Nature 395: 585-588.
CEC 2002a. Commission for Environmental Cooperation, Spring 2002. Trio Newsletter.
CEC 2002b. CEC Council, June 2002. Final Communiqué Ninth Regular Session
CESD 1999. Commissioner for the Environment and Sustainable Development for Canada, 1999. Report to the
House of Commons.
Colborn T, Dumanoski D, Myers JP, 1997. Our Stolen Future.
Environment Canada - U.S. EPA 1996. Atmospheric Deposition of Toxic Substances to the Great Lakes: IADN
Results to 1996; and 2000, The Integrated Atmospheric Deposition Network: What Substances does IADN
Measure and Monitor? http://www.epa.gov/glnpo/air/iadndocs.html. www.msc-smc.ec.gc.ca/iadn
www.epa.gov/glnpo/air/iadndocs.html
Environment Canada 1998. Ecological Monitoring and Assessment Network --- The Northeast States and
Eastern Canadian Provinces Mercury Study: www.eman-
rese.ca/eman/reports/publications/mercury/contents.html.
131
Environment Canada 2002a. Threats to Sources of Drinking Water and Aquatic Ecosystem Health in Canada:
Persistent Organic Pollutants and Mercury, Environment Canada.
Environment Canada 2002b. Environmental Effects Monitoring, Environment Canada. www.ec.gc.ca/eem/.
Evers, Kaplan, Scheuhammer et al. 1998. Geographic trend in mercury measured in common loon feathers and
blood. Environ. Toxicol. Chem, 17: 173-183.
International Joint Commission, 1992, '94, '96, '98, 2000. Biennial Reports on Great Lakes Water Quality.
US EPA, 2000a. Toxics Release Inventory Program
US EPA, 2000b. PBT Program Accomplishments.
US EPA 2000c. Great Lakes Monitoring Lake Michigan Mass Balance. www.epa.gov/glnpo/lmmb/mbc.html.
US EPA, 2000d. Proposed National Action Plan for Level 1 Pesticides, Federal Register November 2000.
WFPHA 2000. World Federation of Public Health Associations, May 2000 Report. Persistent Organic Pollutants
and Human Health.
132
6. FINAL RESULTS AND RECOMMENDATIONS
6.1 Identification of Barriers
This section examines the barriers which exist and which prevent progress in the identification of PTSs and the
evaluation of their levels, trends and consequences as experienced by the environment and humans.
During the review of this report a number of general barriers were identified, including:
· Lack of funding or economic incentives;
· The different governmental models in the 3 countries;
· Lack of communication;
· Lack of incentives and encouragement leadership;
· Different cultural practices;
· Lack of alternatives;
· National legislation, regulatory process that might mean less collaboration ;
· Lack of strategic planning;
· Lack of channels for public participation, lack of access to information.
In the following subsections specific barriers are described.
6.1.1 Lack of information
In Mexico, the occurrence of PTSs and their levels in all environmental compartments are practically unknown.
Regarding the limited data that exist, the quality and adequacy of current databases needs to be assessed using
statistical procedures to validate the information.
In the region as a whole, while there are environmental measurement data on some PTSs of concern, the
majority of the compounds remain unstudied. For "new" chemicals, basic physical properties and toxicology
information is not available as well as analytical methods information.
Apart from some select areas, such as the Great Lakes Basin, large parts of the Region are unstudied. In the
mountainous sub-region bounding the western edge of the North American region and including most of
Mexico, atmospheric, terrestrial and freshwater compartments and pathways should be evaluated in a
comprehensive and systematic way to determine the levels and consequences of PTSs. Work on coastal and
riverine issues is modest. In general, the issue of pesticides in sediments has received little attention compared to
pesticides in water.
Emission inventories are a fundamental component of any examination of the occurrence of PTSs in the
environment. They are also an essential input to many models. Although progress has been made in the last
decade, there are limitations associated with the federal industrial pollutant release and transfer inventories of
the U.S. (TRI) and Canada (NPRI).
· do not encompass all potentially harmful substances;
· do not address all sources from which chemicals of concern move into the environment;
· do not identify all on-site releases and off-site transfers from a facility;
· do not measure releases and transfers-they estimate them;
In Mexico the national inventory is developing but is far from being an efficient and mature archive.
Regional capacity to inventory and assess the potential impact of hot spots, such as orphan sites, contaminated
sediments, waste sites, etc. on a regional basis needs to be improved in order to allow for sound regional
decisions as to the relative significance of these sources relative to the more traditional sources of pollution.
6.1.2 Training
Mexican users and members of civil organizations interested in environmental conservation and protection
usually do not have sufficient training to make use of existing databases. In some cases it is not the data alone
that are missing but also the ability/capacity to gather appropriate data.
133
6.1.3 Research Program
There is a wide range of common scientific issues which cannot be resolved by individual laboratories or by
sub-regional groups. Collaborative tri-national research and monitoring programs on PTSs with a continental
focus are rare. They are needed to ensure that information and expertise on sources, current levels and time
trends, impacts, modeling, etc. are available and are shared.
Uniformity of analytical methodologies is required across the region in order to produce reliable data which are
accepted by the scientific community for the purpose of inter-comparisons and confirmation of results from
participating laboratories.
In general, the long-term research and monitoring programs, needed to assess time trends in PTSs resulting from
UN ECE and UNEP agreements, are not in place. In the Great Lakes Basin the situation is not satisfactory; in
other sub regions it is worse. This is particularly the case for Mexico.
Our capacity to link the chemical measurements to biological effects is weak.
Little is known of the toxicological significance of constant exposure of organisms to low levels of chemicals.
Little is known about the potential effects of mixtures, yet pesticides are often applied as mixtures and certainly
are found in the environment as mixtures.
Progress in developing multi-compartment models has been very encouraging. They provide invaluable
guidance on future monitoring and process research activities; management and/or mitigation measures; and the
sensitivity of individual or linked processes. Their development and application in North America is limited.
The development and application of regionally specific models for Mexico has not occurred. In the case of the
atmosphere, this research gaps means that sources-receptor relationships cannot be easily identified and that
efforts to evaluate control policy options is handicapped.
6.1.4 Capacity
The community education process is weak. Without it the chance of increasing the impetus for
restricting/eliminating the use of PTSs is slim.
Technology transfer/exchange agreements between governments in the region relating to PTSs have not been
initiated despite Mexico's special needs.
There has been but a modest effort made to promote compliance with national programs and regulations,
including voluntary measures, in the three North American countries and to promote harmonization, to the
extent possible, across the region as demonstrated in the CEC's North American Regional Action Plans.
6.2 Priorities and Recommendations for Future Activities
During the review of this report the following key priority areas were identified.
1. Emerging
PTSs:
· North America should leverage existing capacity and focus on research/assessment of emerging PTSs.
2. Knowledge and Technology Transfer in the Regions:
· Networks of scientists working together to address
o Scientific exchanges
o Emerging contaminants
o Technology transfer
o Information exchange and the aligning the procedures for assessment
3. Ratification of the Stockholm Convention in North America:
· Tri-national ratification
· Develop national implementation plans with milestones (e.g. US manufacturers no longer exporting
POPs substances)
134
o Need program evaluation criteria
o Need clear roles and responsibility
4. Addressing research and monitoring needs and data gaps related to:
· Sources and hot spots, particularly in Mexico
· The physical/chemical properties of PTSs,
· Emission inventories including the quantification of sources, sinks and reservoirs and movements of
PTSs into and out of the region
· Spatial variability of PTSs levels for a larger number of chemicals
· "Cradle to grave", pathway analysis of each substance from its production to its final disposal
· Exposure pathways in food, agriculture and aquaculture for exposed populations and subsistence food
production systems.
· Presence of PTSs in water bodies and runoff from agricultural fields
· Open Burning including developing a definition, taking a leadership role and encouraging a global
campaign to devise new waste management strategies including waste minimization and waste
prevention.
5. Improving
the
Mexican Infrastructure:
· Complete a national information structure for:
o Emissions, sources, sinks and reservoirs
o Areas with severe degradation
o Monitoring of contaminants in various compartments
· Develop analytical capacity to the point where comparable data and information exists among the
three countries.
6. Creating a North American regional pollution prevention program:
· Include a framework for public education, technology transfer, and education in best practices.
· Encourage communication between existing infrastructures.
· A specific strategy needed for Mexico that will use and adopt technologies and practices available in
Canada and the U.S.
· In areas where we have confidence now, take action to control and reduce.
7. Public education in North America involving:
· NGO's
· Appropriate levels of government
· A focus on children, women and indigenous peoples.
8. Improving public participation and input into decision-making related to PTSs
135
ANNEX: SCORING FOR PRIORITISING PTS FOR SOURCES,
ENVIRONMENTAL LEVELS, EFFECTS AND DATA GAPS
Instructions:
1) Chemicals to be grouped by matrix (sources, environmental conc., etc.) and by score. There is no total
score for any chemical.
2) An associated column for data gaps is attached to each matrix. For example, Sources has an
accompanying column to score the degree of data gaps experienced for Sources.
3) A short summary with representative, specific data must be used to justify the score given. Use <50
words.
4) All chemicals selected for the study must have a score for each category
5) The guidelines attached provide a qualitative measure for scores. Scores are measured as follows:
Scores: Score = 0 chemical is of no concern/ supportive data is collected
Score = 1 chemical has local concern/ supportive data is limited
Score = 2 chemical has regional concern/ supportive data is lacking
NB. The score given for a matrix on a chemical does not have to be the same score for data gaps on that matrix
Table 2.
Guidelines for Scoring Issues Associated with Each Chemical
Issue
Score 0 =
Score 1 =
Score 2 =
No concern
Local concern
Regional concern
Sources of the
No evidence of production Evidence of limited
Major production of
Chemical
or product contamination
production
chemical for local and
No evidence of air
Presence of small sources
export use.
emissions
with possible emissions
Chemical evident as
(e.g.,small incineration
contaminant in large scale
No evidence of emissions
from solid residues
plants or bleached
production of other
kraft/pulp mills using
chemicals
No evidence of chemical
chlorine);
stockpiled
Known emission of
Some limited evidence of
chemical from large scale
No evidence of chemical
releases but on a small scale incinerators or chlorine
being contaminant in
invoking local concerns
bleaching of pulp or other
production of other
Some use of the chemical
related combustion
chemicals
locally
facilities
No evidence of use of the
Evidence of leakage from
chemical
Over time, levels remain
below threshold or are
major stockpiles of the
No evidence of release
decreasing
chemical poorly packaged
from liquid effluent
Use of chemical in
Large-scale use of the
agriculture or industry sub-
chemical throughout the
regionally
region
Evidence of limited
Spatial and/or temporal
stockpiles of the chemical
trends increasing regionally
from levels above threshold
Increasing spatial and/or
temporal trends from
localised levels below
threshold
136
Environmental
No known or historical
Chemical contaminants are
Chemical contaminant is
Levels of the
levels of chemical
detectable in the
analysed repeatedly well
chemical
contaminant in the
environment but below
above threshold limits in
environment except
threshold limits defined for
the environment defined
background levels of
the country or sub-region
for a country or region
naturally occurring
Chemical contaminants are
Known contamination of
substances
detectable from fish,
fish, wildlife, foodstuff or
No available data to
wildlife, foodstuff or
humans at levels far
quantify evidence of the
human samples but below
exceeding the threshold
chemical found in fish,
threshold limits established
established for the country
wildlife animal or human
for the country or sub-
or region
tissue
region
Spatial and/or temporal
Over time, levels remain
trends increasing from
below threshold or are
levels above threshold
decreasing
Increasing spatial and/or
temporal trends from levels
below threshold
Ecotoxicological
No fisheries closures or
Inconclusive evidence of
Public health and public
Effects from
advisories due to chemical limited fish or wildlife
awareness of food/fisheries
exposure to the
pollution
mortality events on a local
contamination problems
chemical
No incidence of
or sub-regional scale
with associated reduction in
food/fisheries product
Temporal trend shows
the marketability of such
tainting
constant or decreasing
products either through the
imposition of limited
No unusual fish or
effect of chemical
advisories or by area
wildlife mortality events
closures
Large-scale mortalities of
aquatic or wildlife species
Temporal trend showing
increase in effects of
chemical regionally
No indication of any ill
Odd incidence of ill effects
Indications of health effects
effects from exposure to
that may be related to
resulting from use of
Human Effects
the chemical
exposure to the chemical
pesticide/industry chemical
from exposure to No correlation between
Evidence of localised effects Wide spread health effects
the chemical
human diseases and
from spot exposure to the
from involuntary exposure
chemical exposure
chemical
to the chemical
Temporal trend shows
Temporal trend showing
constant or decreasing
increase in effects of
effect of chemical locally
chemical regionally
Data gaps
Full data sets established
Limited data available
Sparse data available
Evidence complete
Minimum data required to
Data unreliable
Ongoing monitoring data
confirm findings
No data available
available
Data available conflicting
Only historical data (>20
Further monitoring data
years) available
required on a wider scale
Limited data available
Limited anecdotal evidence
shows major concern
of local human and
Widespread anecdotal
environmental effects
evidence of human and
environmental effects
137
Region II North America RBA PTS
Chemicals Scoring Worksheet Sources
Chemical
Sources Data
Gaps
Comments
US CA ME US CA MX
Aldrin
- US data from TRI, not listed in CA
1 0 0 0 0 0
- not listed in Mexico since banned 1991
Chlordane
- US data from TRI, not listed in CA
1 0 1 0 0 1
- listed in Mexico, no data, imported into Mx
DDT
- no data in TRI or NPRI
0 0 1 0 0 1
- restricted in Mx since 1991, imported into Mx
Dieldrin
- conversion from Aldrin, not listed in TRI or NPRI
1 0 0 0 0 0
- not listed in Mexico since banned 1991
Endrin
- not listed in US or CA, converted from aldrin
1 0 0 0 0 0
- not listed in Mexico since banned 1991
Heptachlor
- US data from TRI, not listed in CA
1 0 1 0 0 1
- listed in Mexico, imported into Mx
HCB
- US data from TRI, listed in CA, no data yet
1 1 1 1 1 1
- listed in Mexico, no data, imported into Mx
Mirex
- not listed in TRI or NPRI
0 0 1 0 0 1
- not listed in Mexico since banned 1991, imported into
Mx
Toxaphene
- US data from TRI, not used, produced or listed in CA
1 0 1 0 0 1
- not listed in Mexico, imported and possible illegal use
PCBs
- US data from TRI, CA gov wide PCB inventory data,
2 2 1 1 1 1 lake sediment cores, data from INE in Mx
Dioxins
- US data from TRI, listed in CA, EC website
2 2 1 1 1 1
- no control program in Mexico
Furans
- US data from TRI, listed in CA, no data yet
2 2 1 1 1 1
- no control program in Mexico
138
HCH
- not listed in TRI or NPRI, some data exists
2 2 2 1 1 1
- Mx AMIFAC data
PCP
- US data from TRI, not listed in CA, some data from HC
2 2 1 1 1 1
- not listed in Mx since restricted 1991, produced in Mx
PAHs
- US data from TRI, listed in CA, no data yet
2 2 0 1 1 2
- no control program in Mexico
Org. Mercury
- US data from TRI and USEPA, listed in NPRI, no data
2 2 1 1 1 2
Compds.
avail, some data from EC, imported into Mx
Org. Tin
- not listed in TRI or NPRI, some data on manufacturing
1 1 0 1 1 2
Compds.
sources, no control program in Mexico
Org Lead
- US data from TRI, listed in NPRI, no data avail, some
2 2 0 1 1 2
Compds.
data from EC, no control program in Mexico
PBDE
- not listed in TRI or NPRI, some data on combustion
1 1 0 1 1 2 sources, not listed in Mx, production volumes in Mx
Phthalates
- US data from TRI, listed in CA, no data yet
2 2 0 1 1 2
- not listed in Mx
Endosulphan
- not listed in TRI or NPRI, US data from ASTDR
2 2 1 1 1 2
- listed in Mexico, no data, imported into Mx
Atrazine
- ASTDR, USEPA data, LaMP data in CA
2 1 1 0 0 1
- listed in Mexico, AMIFAC data, imported into Mx
Chlordecone
- not listed in TRI or NPRI, banned in US
0 0 0 0 0 0
- not listed in Mexico since banned 1991
Octylphenols
- not listed in TRI or NPRI, some data from CEPA
1 2 0 1 1 2
- no control program in Mexico
Nonylphenols
- not listed in TRI, listed in NPRI, no data yet, some data
2 2 0 1 1 2 from EC, no control program in Mexico
Perfluorooctanyl
- not listed in TRI or NPRI, no control program in Mexico
1 1 0 1 1 2
sulfonate (PFOS)
(info chapter 3)
Chlorinated
- data in TRI, Env Can, sources listed in manufacturing,
1 1 1 1 1 2
Paraffins
imported into Mexico
139
Region II North America RBA PTS
Chemicals Scoring Worksheet Environmental Levels
Chemical
Env. Levels
Data Gaps
Comments
US CA ME US CA MX
Aldrin
- in sediments in Mx,
1 1 1 0 0 1
Chlordane
- data from IADN stations Great Lakes for CA, US, also found
2 2 0 1 1 2 in Osprey eggs, human adipose tissue
DDT
- date on herring gulls in Lake Michigan, terns in Lake Ontario,
USEPA studies in Grt Lakes, IADN stations, aquatic food webs,
1 1 2 0 0 1 Osprey eggs and body tissue, eagle eggs, Canadian Lakes,
various fish. lake sediment cores in US and CA, human breast
milk, Baltic seals, mink, human serum, lagoon sediments Mx
Dieldrin
- some data from USEPA studies in Grt Lakes, IADN Stations,
1 1 0 1 1 2 Herring Gull eggs, Osprey eggs and body tissue, human milk
Endrin
- rarely found in adipose tissue and human breast milk
1 1 0 0 0 1
Heptachlor
- Herring gull eggs, human adipose, human breast milk, lagoons
1 1 1 0 0 1 in Mx
HCB
- IADN Stations, Herring gull eggs, terns, oysters Mx
1 1 0 1 1 2
Mirex
- fish in Bay of Quinte, CA, Great Lakes, IADN Stations,
1 1 0 0 0 2 Herring gull eggs
Toxaphene
2 2 0 1 1 2 - Great Lakes, fish advisories, various fish in various CA lakes,
sediments
PCBs
- terns in Lake Ontario, Lake Trout, Salmon in Grt Lakes, IADN
Stations, Grt Lakes Region in air and water, Beluga whales,
2 2 2 1 1 2 Herring gull eggs, shellfish, fish eating birds, Osprey eggs,
various fish in various lakes in CA, Polar Bears, Baltic Seals,
mink, human serum, human breast milk, human adipose tissue,
oysters Mx increasing trend
Dioxins
- USEPA Grt Lakes data, fish consumption advisories Grt
1 1 0 1 1 2 Lakes, Herring gull eggs, Osprey eggs, sediments, overall levels
decreasing
140
Furans
- USEPA Grt Lakes data, fish consumption advisories Grt
1 1 0 1 1 2 Lakes, Herring gull eggs, Osprey eggs, sediments, overall levels
decreasing
HCH
- IADN Stations, lake trout, human serum, human breast milk,
2 2 2 1 1 1 human adipose tissue, lagoon sediments Mx, shrimp Mx
PCP
- blood of marine mammals and humans
2 2 0 1 1 2
PAHs
- USEPA data from Grt Lakes, IADN Stations, fish
2 2 1 1 1 1
Org. Mercury
- USEPA Great Lakes Data, fish advisories, thick billed murre
Compds.
2 2 0 1 1 2 eggs, sediment cores, Herring gull eggs, Osprey eggs, various
fish in various lakes in CA, eagles, human hair, human serum
Org. Tin
- human adipose tissue
1 1 0 2 2 2
Compds.
Org Lead
- USEPA Great Lakes data, IADN Stations, eagles, other birds
Compds.
1 1 2 1 1 2 and eggs, human serum, bone, oysters Mx, levels decreasing in
CA and US
141
Chemical
Env. Levels
Data Gaps
Comments
US CA ME US CA MX
PBDE
- human breast milk, fish, air, umbilical cord blood,
2 2 0 1 1 2 ringed seals, gull eggs,
Phthalates
0 0 0 2 2 2
Endosulphan
- lake trout, lake water, lagoons in Mx
2 2 1 1 1 1
Atrazine
- various lakes in CA and US, atmosphere, sediments,
2 2 0 1 1 2 lake water
Chlordecone
- banned pesticide
0 0 0 1 1 1
Octylphenols
- sediments Mx
0 0 1 2 2 2
Nonylphenols
- sediments, carp
1 1 1 1 1 2
Perfluorooctanyl
- Polar Bears, fish eating birds, human serum, seals,
2 2 0 1 1 2
sulfonate (PFOS)
minks, herring gulls, cormorants, bald eagles
Chlorinated
- various lakes in CA and US, sediment core samples,
1 1 0 1 1 2
Paraffins
fish
142
Region II North America RBA PTS
Chemicals Scoring Worksheet Ecotoxic Effects
Chemical
Ecotoxic
Data Gaps Comments
Effects
Aldrin
- toxic to aquatic organisms
1 1
Chlordane
- highly toxic to aquatic organisms, moderately toxic to
1 1
mammals
DDT
- data from USEPA studies in Great Lakes, eggshell thinning,
1 1
decreased reproduction, acutely toxic to birds, moderately toxic
to mammals
Dieldrin
- some data from USEPA studies in Great Lakes, adverse
1 1
reproductive effects in bald eagles, - toxic to aquatic organisms
and mammals
Endrin
- toxic to aquatic organisms and mammals
1 1
Heptachlor
- highly toxic to aquatic organisms, moderately toxic to
1 1
mammals
HCB
- low acute toxicity in rats, shows liver effects at low levels
2 1
Mirex
- moderate toxicity in mammals, toxic to fish
1 1
Toxaphene
- highly toxic to fish, carcinogenic in mice
1 1
PCBs
- cross bill deformity in terns, decreased reproductive success,
2 1
club feet, extra digits in birds, eye and skeletal deformities, low
acute toxicity in mammals, possible carcinogen
Dioxins
- dermal toxicity, immunotoxicity, reproductive effects,
2 1
endocrine disruption, carcinogen
Furans
- dermal toxicity, immunotoxicity, reproductive effects,
2 1
endocrine disruption, carcinogen
143
HCH
- moderately toxic for invertebrates and fish, moderate acute
1 1
toxicity in mice, possible endocrine disruptor
PCP
- acute toxicity to aquatic organisms, moderate to high acute
1 2
toxicity in mammals
PAHs
- cancer in fish, may cause impaired physiology in mudpuppies,
2 1
acute toxicity of low PAHs is moderate, and high for high
PAHs
Org. Mercury
- reproductive effects
2 1
Compds.
Org. Tin
- causes immune system disorders, endocrine system disorders
Compds.
1 1
and CNS disorders in lab animals, skin and eye irritant,
moderately toxic to aquatic organisms
Org Lead
- acute toxicity is moderate in mammals and high in aquatic
1 1
Compds.
organisms
144
Chemical
Ecotoxic
Data
Comments
Effects
Gaps
PBDE
- lower congeners likely to be carcinogenic, endocrine
1 1
disruptors or neurodevelopmental toxicants
- low acute toxicity in rats
Phthalates
- acute toxicity is low, high amounts can damage liver and
1 2
kidney
Endosulphan
- toxic in birds and rats
1 1
Atrazine
- decreased reproduction, affects sexual reproduction in frogs
1 1
Chlordecone
- nervous system, skin, liver, reproductive, kidney effects,
1 1
carcinogenic in mice and rats
Octylphenols
- possible endocrine disruptor, highly toxic to some fish species
1 2
Nonylphenols
- possible endocrine disruptor
1 2
Perfluorooctanyl
- interferes with fatty acid binding, may cause cancer, levels
2 1
sulfonate (PFOS)
increasing, moderate acute toxicity to aquatic organisms
Chlorinated
- acute toxicity low in mammals, toxic with long term exposure
Paraffins
1 2
145
Region II North America RBA PTS
Chemicals Scoring Worksheet Human Health Effects
Chemical
Human
Data Gaps Comments
Effects
Aldrin
- metabolized to dieldren, may effect reproduction and
1 1
development, immune system and may be carcinogenic
Chlordane
- may effect reproduction and development, immune system
1 1
and may be carcinogenic
DDT
- some data from USEPA Grt Lakes, lipophilic and toxic, may
1 1
effect reproduction, immune system, adrenal and thyroid
glands, endocrine system, found in breast milk, adipose tissue
Dieldrin
- some data from USEPA Grt Lakes, may effect reproduction
1 1
and development, immune system and may be carcinogenic,
found in breast milk
Endrin
- found rarely in breast milk, low conc in adipose
1 1
Heptachlor
- found in breast milk, adipose tissues, decreasing levels
1 1
HCB
- may effect reproduction and development, immune system,
2 1
thyroid and may be carcinogenic
Mirex
- may effect reproduction and development, immune system
1 1
and may be carcinogenic
Toxaphene
- may effect reproduction and development, immune system ,
1 1
adrenal and thyroid glands, skeletal system and may be
carcinogenic
PCBs
- may effect reproduction and development, immune system ,
2 1
thyroid gland, skeletal system and may be carcinogenic, causes
low birth weights
Dioxins
- found in blood and adipose of US residents
2 1
- may effect reproduction and development, immune system,
thyroid gland, skeletal system and may be carcinogenic and
cause porphyria
Furans
- see dioxins
2 1
146
HCH
- may effect reproduction and development, immune system,
1 2
thyroid, adrenal and may be carcinogenic, found in serum,
adipose US
PCP
- found in urine and serum Us
0 2
PAHs
- possible carcinogen to humans
2 1
Org. Mercury
- neurotoxin, mental retardation, cerebral palsy, deafness,
Compds.
2 1
blindness, can effect cardiovascular system, cancer, mercury
levels increasing
Org. Tin Compds.
1 2
- may effect reproduction, development, the nervous system and
the immune system
Org Lead
- abdominal pain, vomiting, diarrhea, convulsions, coma or
Compds.
1 1
even death, chronic exposure leads to anemia, impaired mental
function, impaired kidney function, and neurological damage in
children, lead levels decreasing
PBDE
- dioxin like toxicity, effects cytochrome P450 system, may
2 1
cause thyroid effects, found in high levels in human breast milk,
found in umbilical cord blood
Phthalates
0 2
Endosulphan
- may effect reproduction and development, immune system
1 1
and may be carcinogenic
Atrazine
0 2
Chlordecone
- banned pesticide, exposed workers show nervous system,
1 1
skin, liver, male reproductive system effects
Octylphenols
0 2
Nonylphenols
0 2
Perfluorooctanyl
- interferes with fatty acid binding and transport, decreases
sulfonate (PFOS)
2 1
cholesterol, inhibits cell-to-cell communication, potentially
causes cancer, and has reproductive and developmental effects
Chlorinated
Paraffins
0 2
147
148




United Nations
Environment Programme
Chemicals
Nor
North America
th America
REGIONAL REPORT
RBA PTS REGIONAL REPOR
Regionally
Based
Assessment
T
of
Persistent
Available from:
UNEP Chemicals
11-13, chemin des Anémones
CH-1219 Châtelaine, GE
Switzerland
Phone : +41 22 917 1234
Fax : +41 22 797 3460
Substances
E-mail: chemicals@unep.ch
December 2002
http://www.chem.unep.ch
UNEP Chemicals is a part of UNEP's Technology, Industry and
Printed at United Nations, Geneva
Economics Division
GE.03-00147January 2003500
UNEP/CHEMICALS/2003/2
G l o b a l E n v i r o n m e n t F a c i l i t y








